It takes two to tango ethical dilemmas of




























- Slides: 38

It takes two to tango: ethical dilemmas of health professionals and therapeutic goods industry Dr Ken Harvey MB BS, FRCPA http: //www. medreach. com. au AMSA GHC, Hobart, September 12 -15, 2013

Talk outline • Disclosure of interests • Snapshot of current issues • WHO resolutions and QUM policy • Self-regulatory Codes – Pros and cons • Unethical behaviour that led to: A fundamental change in the relationship between industry and medical professionals is needed. – U. S. Physicians Payment Sunshine Act – Therapeutic Goods Amendment (Pharmaceutical Transparency) Bill 2013 – Medicines Australia current Code revision – AHPRA Code of Conduct • Conclusion: It takes two to tango. 2

Disclosure of interests • Member: – WHO Ethical Criteria for medicinal drug promotion. – Therapeutic Guidelines Limited. – PHARM Committee that devised the Quality Use of Medicines plank of Australian Medicines Policy. • Consumer rep (Choice, CHF): – Government Working Group on Promotion of Therapeutic Products. – TGA Transparency Review Panel. – TGA Working Group on Regulatory Framework for Complementary Medicines. – Government Natural Therapy Review Advisory Committee.

Snapshots of current issues Mea culpa: are multi-billion dollar fines forcing drug companies to clean up their act? BMJ 2012; 345 (Published 18 July 2012) How Drug Company Money Is Undermining Science November 21, 2012 The pharmaceutical industry funnels money to prominent scientists who are doing research that affects its products--and nobody can stop it

World Health Assembly 2007 Resolution 60. 16: – Wishing to promote evidence-based rational use of medicines by providers and consumers; • URGES Member States to: – Develop and implement programmes that will provide independent, non-promotional information about medicines; – Enact new, or enforce existing, legislation to ban inaccurate, misleading or unethical promotion of medicines; – Monitor promotion. 5

Quality Use of Medicines Policy Strategies • Policy development and implementation • National facilitation and coordination • Independent objective information • Ethical behaviour • Education and training • Interventions • Evaluation 6

Independent objective information Prescription medicines 7

Independent objective information Prescription medicines Bad research rising: The 7 th Olympiad of research on biomedical publication By Hilda Bastian | September 8, 2013 http: //www. peerreviewcongress. org/index. html 8

Ethical behaviour “There is an integrated three-tier system of controls for the advertising of therapeutic goods in Australia”. http: //www. tgacrp. com. au/ – Self-regulation (promotion to health professionals) • Industry Codes and complaints panels in nine sectors: Medicines Australia, GMIA, ASMI, CHC, MTAA, Aus. Bio. Tech, IVD Australia, ADA, ACCORD. • Medical Board and health professional Codes. – Co-regulation (promotion to consumers) • Therapeutic Goods Advertising Code (TGAC), Complaints Resolution Panel (CRP) and TGA. – Regulation (legislation) • Therapeutic Goods Act 1989 (TGA) and Trade Practices Act, 1974 now renamed the Competition and Consumer Act 2010 (ACCC). 9

Self-regulation: Pros “The Australian Government’s preference is to maintain an emphasis on self-regulation”. TGA reforms: A blueprint for TGA’s future. December 2011. • The government does not pay the cost (however, the cost to industry is passed on to consumers regardless); • May be more flexible and less intrusive than government legislation / regulation; • Ownership of a code may produce a stronger commitment for members to comply; • Complaint handling procedures under a self-regulatory code can be more cost effective, time efficient and user friendly in resolving complaints than government bodies. 10

Self-regulation: Cons There are nine therapeutic goods industry self-regulatory codes 11

Self-regulation: Cons • Code content, monitoring, complaint procedures and transparency vary across industry sectors (“not a level playing field”). • Codes often lag behind consumer and health practitioner views due to the absence of external stakeholders. • Codes also lag behind the views of more progressive companies because of the need for revisions to be approved by a majority of member companies. • Codes don't apply to non-members; a major problem in some sectors of therapeutic goods industry. • Numerous sector based industry Codes make it difficult to 12 know where to send complaints.

Self-regulation: Cons • Following media stories about a Sigma cruise and unethical conduct by medical device sponsors Parliamentary Secretary for Health Mark Butler said: – “The Government is pursuing a level playing field on marketing obligations within therapeutic goods industry”. 13

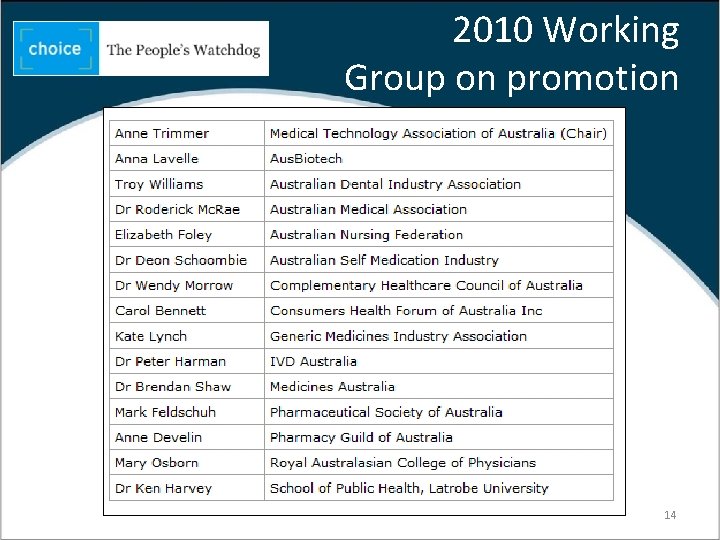
2010 Working Group on promotion 14

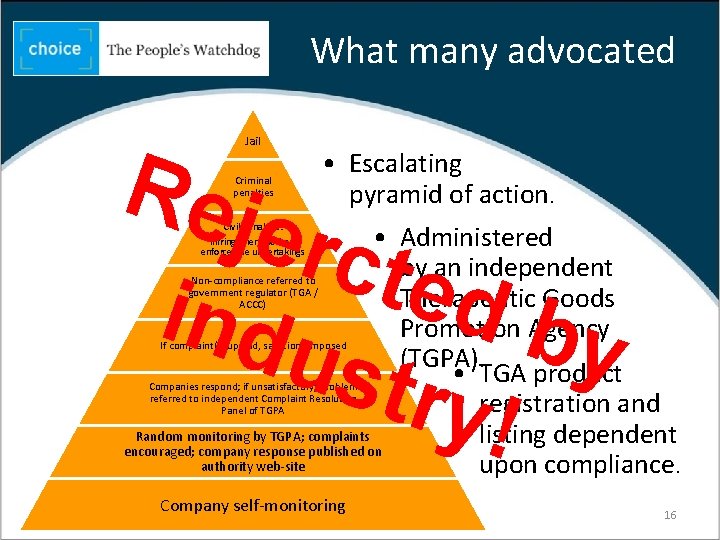
What many advocated • One Code, one efficient complaint (and appeal) system and one set of effective sanctions applicable to all therapeutic claims and promotional activities regardless of the industry sector, media or target. 15

What many advocated Rej erc t e db ind y ust ry! Jail Criminal penalties • Escalating pyramid of action. • Administered by an independent Therapeutic Goods Promotion Agency (TGPA). • TGA product registration and Random monitoring by TGPA; complaints listing dependent encouraged; company response published on authority web-site upon compliance. Civil penalties: infringement notices, enforceable undertakings Non-compliance referred to government regulator (TGA / ACCC) If complaint(s) upheld, sanctions imposed Companies respond; if unsatisfactory; problem referred to independent Complaint Resolution Panel of TGPA Company self-monitoring 16 16

2010 Working Group Report • Working Group on Promotion of Therapeutic Products (to health professionals) delivered its report to then Parliamentary Secretary for Health Catherine King on 18 March 2011. • It recommended a high level statement of principle: – the Australian therapeutic products industry promotes the concept of good health incorporating the quality use of therapeutic products based on genuine consumer health needs and supported by the ethical conduct of all parties. • Common coverage of operational areas, such as industrysponsored educational events, conduct of representatives, hospitality and entertainment, and social media. 17

2010 Working Group Report • Common governance areas for effective implementation: – Education on the code’s operation; – Monitoring of compliance with the code; – Enforcement of the code in response to a complaint or a breach; – Sanctions to support the enforcement (at a level that deters non-compliance). • The government make compliance with a Code a condition of market authorisation (to address the problem of non-members). • The latter was rejected by government which preferred voluntary compliance! 18

Evaluating voluntary Code sign-up Kate Lynch (CEO, GMi. A) noted: • GMi. A already provides an option for nonmembers to voluntarily sign up to the GMi. A Code without joining the association. • Despite pro-actively advocating this facility no company has taken up the option. • Non-members have stated that they already adhere to the Code and do not see a need to make any formal declaration. 19

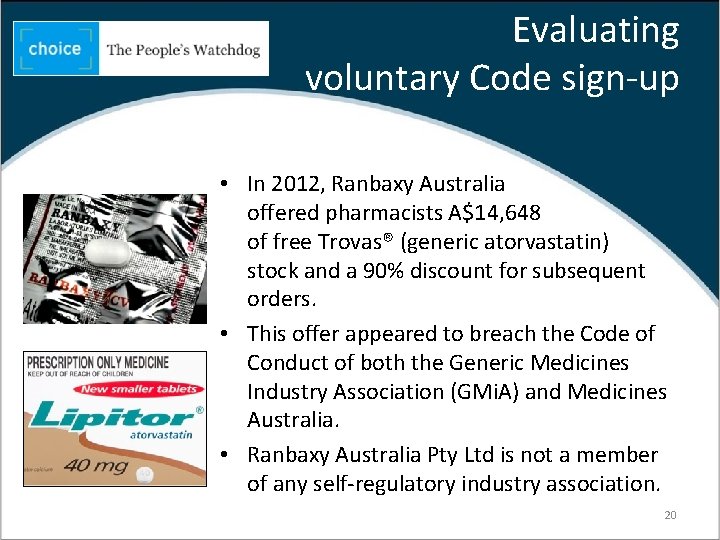
Evaluating voluntary Code sign-up • In 2012, Ranbaxy Australia offered pharmacists A$14, 648 of free Trovas® (generic atorvastatin) stock and a 90% discount for subsequent orders. • This offer appeared to breach the Code of Conduct of both the Generic Medicines Industry Association (GMi. A) and Medicines Australia. • Ranbaxy Australia Pty Ltd is not a member of any self-regulatory industry association. 20

Evaluating voluntary Code sign-up http: //www. pharmainfocus. com. au/news. asp? newsid=5482 21

2013 -16 Codes of Conduct Advisory Group • Contains representatives from industry associations, health professional and consumer organisations together with Do. HA responsible for: Chair: Prof Lloyd Samson – an independent review of the uptake of the 2010 Working Group’s high level principles; – development of shared information systems and a common complaints portal; – alignment of industry and health professional codes; – mechanisms to improve the coverage of codes of conduct; – an independent evaluation of the effectiveness of the overall self-regulatory framework; – A budget of $1. 4 million from 2012 -16. 22

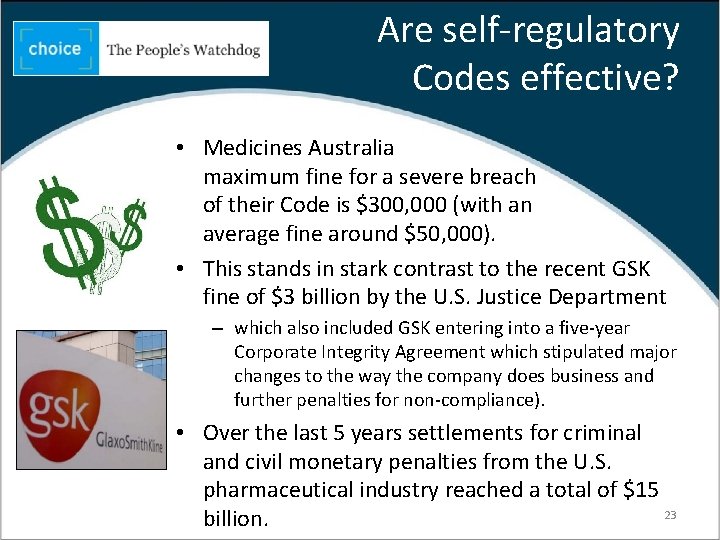
Are self-regulatory Codes effective? • Medicines Australia maximum fine for a severe breach of their Code is $300, 000 (with an average fine around $50, 000). • This stands in stark contrast to the recent GSK fine of $3 billion by the U. S. Justice Department – which also included GSK entering into a five-year Corporate Integrity Agreement which stipulated major changes to the way the company does business and further penalties for non-compliance). • Over the last 5 years settlements for criminal and civil monetary penalties from the U. S. pharmaceutical industry reached a total of $15 23 billion.

U. S. Justice Department Fines 24

Unethical conduct • Trials manipulated; negative results suppressed. • Journal articles “ghost-written”. • Off-label promotion. • Well paid, but undeclared, medical opinion leaders used to promote company products (educational mercenaries). • Excessive hospitality, kickbacks, bribery. • Consumer groups manipulated. • Spurious patents and legal challenges to delay the 25 entry of generics. 25

It takes two to tango 26

The need for disclosure • A recent editorial in the British Medical Journal reported that a US Senate Finance Committee investigation has found the medical device company Medtronic was “heavily involved in drafting, editing, and shaping the content of medical journal articles authored by its physician consultants, ” who were paid hundreds of millions of dollars by the company through royalties and consulting fees. • The Committee Chairman, Senator Max Baucus, said Medtronic’s actions had “violated” patients’ trust. • The president of the North American Spine Society, said, “If surgeons had known that the lead authors of the 13 original studies on In. Fuse (the Medtronic product) had received payments ranging from $1. 7 m to $64 m from Medtronic and that its marketing employees were co-authors and co -editors, would they have been as eager to use this product on their patients? ” 27 http: //www. bmj. com/content/345/bmj. e 7299

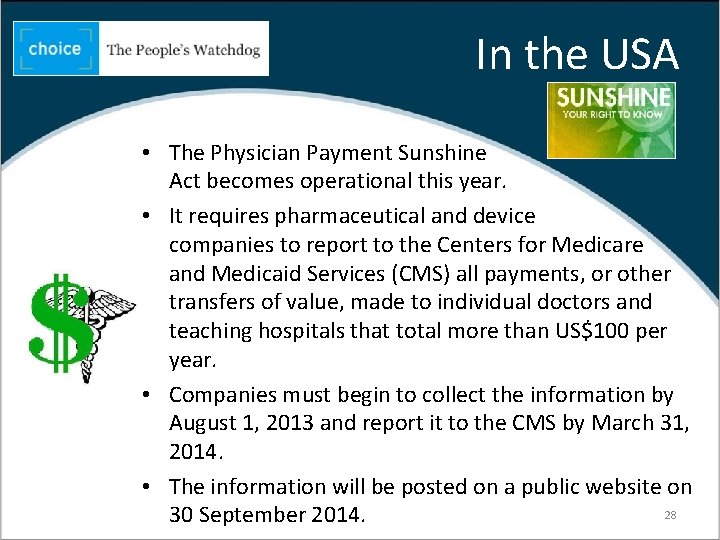
In the USA • The Physician Payment Sunshine Act becomes operational this year. • It requires pharmaceutical and device companies to report to the Centers for Medicare and Medicaid Services (CMS) all payments, or other transfers of value, made to individual doctors and teaching hospitals that total more than US$100 per year. • Companies must begin to collect the information by August 1, 2013 and report it to the CMS by March 31, 2014. • The information will be posted on a public website on 28 30 September 2014.

In Australia • • • Many consumer and health professional groups argued for full disclosure of payments made to individual healthcare professionals in the 16 th Edition (2009) Code revision and again in the 17 th Edition (2012) Code revision. The 17 th Code Edition incorporated aggregate disclosure of payments, not individual. MA set up a Transparency Working Group to advise how individual disclosure might be implemented. A report has now been produced but there was no agreement on the amount of money that would trigger individual disclosure. Consultation with MA members and other stakeholders will continue with (hopefully) the adoption of individual payment disclosure in new Code revision in late 2014 with possibly payments being recorded from Jan 1, 2015 and public reporting in 2016 (compared to 2014 in the U. S. ). 29

In Australia • This bill proposed by Senator R Di Natale would have replaced Medicines Australia Code with legislation that set stringent restrictions on the interactions between pharmaceutical companies and physicians. • In June 2013, the Finance and Public Administration Legislation Committee recommended that the bill not be passed. Unlike the U. S. , self-regulation was thought to be more appropriate in Australia. 30

MA Code review There will soon be a call for submissions on the options in discussion paper and also forums where all stakeholders (including AMSA) may come together and discuss the transparency model. 31

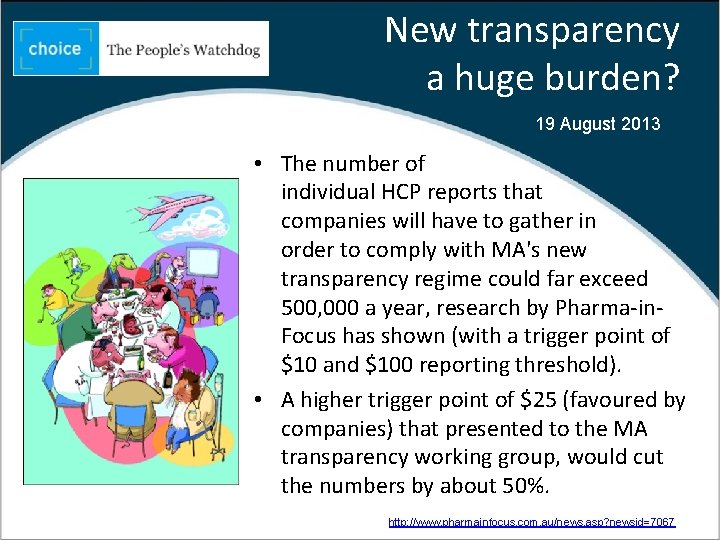
New transparency a huge burden? 19 August 2013 • The number of individual HCP reports that companies will have to gather in order to comply with MA's new transparency regime could far exceed 500, 000 a year, research by Pharma-in. Focus has shown (with a trigger point of $10 and $100 reporting threshold). • A higher trigger point of $25 (favoured by companies) that presented to the MA transparency working group, would cut the numbers by about 50%. http: //www. pharmainfocus. com. au/news. asp? newsid=7067

AHPRA code of conduct for doctors in Australia 8. 11. 4 Recognising that pharmaceutical and other medical marketing influences doctors, and being aware of ways in which your practice may be being influenced. 8. 11. 5 Recognising potential conflicts of interest in relation to medical devices and appropriately managing any conflict that arises in your practice. 8. 11. 6 Not asking for or accepting any inducement, gift or hospitality of more than trivial value, from companies that sell or market drugs or appliances that may affect, or be seen to affect, the way you prescribe for, treat or refer patients. 8. 11. 8 Not offering inducements to colleagues, or entering into arrangements that could be perceived to provide inducements. http: //www. medicalboard. gov. au/Codes-Guidelines-Policies. aspx 33

Monitoring promotion 34

It takes two to tango 35

It takes two to tango The Pharma Phacts Pledge I pledge to accept no money, gifts, or hospitality from the pharmaceutical industry; to seek unbiased sources of information and not rely on information disseminated by drug companies; and to avoid conflicts of interest in my medical education and practice. 36 http: //www. healthyskepticism. org/pharmaphacts/

In conclusion: One book to read 37

In conclusion: Organisations to join • Health Action International – http: //www. haiweb. org/ • Healthy Skepticism – http: //www. healthyskepticism. org/global/ • Friends of Science and Medicine – http: //www. scienceinmedicine. org. au/ • Choice Consumer Action – http: //www. choice. com. au/consumeraction. aspx 38
 School social work ethical dilemmas
School social work ethical dilemmas Ethical dilemma example
Ethical dilemma example 5 steps in resolving ethical dilemmas
5 steps in resolving ethical dilemmas Engineering ethical dilemmas
Engineering ethical dilemmas What is ethical reasoning
What is ethical reasoning Cpa ethical dilemmas
Cpa ethical dilemmas Historical ethical dilemmas
Historical ethical dilemmas Ethical dilemmas in early childhood education
Ethical dilemmas in early childhood education Moral dilemmas in the crucible
Moral dilemmas in the crucible Leadership dilemmas grid solutions
Leadership dilemmas grid solutions Perbedaan ethical dilemma dan ethical lapse
Perbedaan ethical dilemma dan ethical lapse Army ethical lenses
Army ethical lenses Csr and business ethics
Csr and business ethics The perceived relevance or importance of an ethical issue
The perceived relevance or importance of an ethical issue It takes two stuck in slide
It takes two stuck in slide It takes two
It takes two Tango dance time signature
Tango dance time signature Tango scada
Tango scada Electone tango 2p
Electone tango 2p Spanien är ett land där man dansar tango
Spanien är ett land där man dansar tango Tango netball club
Tango netball club Tango control system
Tango control system Tango switch
Tango switch Local guide program
Local guide program Cuestionario sobre tango de bing
Cuestionario sobre tango de bing Cuestionario sobre tango de bing
Cuestionario sobre tango de bing Mecanism
Mecanism Celemi tango
Celemi tango Tango argentin sensuel
Tango argentin sensuel Tango van het blote kontje
Tango van het blote kontje Tango
Tango Tango
Tango Tango sin and passion
Tango sin and passion Dátil
Dátil Tango pooling
Tango pooling Tango poema bailado
Tango poema bailado Tango controls
Tango controls Tango in progress roma
Tango in progress roma Pam jogged up a hill at 6 km/h
Pam jogged up a hill at 6 km/h