Introduction What is energy ENERGY ENERGY Sound is









- Slides: 14

Introduction: What is energy? ENERGY

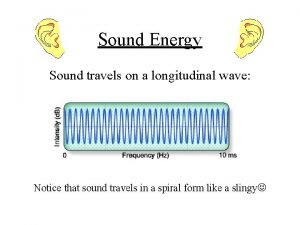
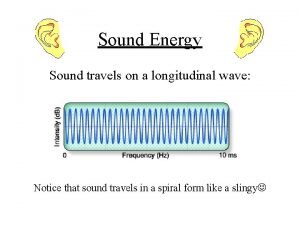
ENERGY? ? Sound is energy!

ENERGY? ? Light is energy!

ENERGY? ? ? Chemical Reactions are Energy Chemical Reaction

ENERGY? ? ? Heat is energy

BONDS BREAK AND FORM DURING CHEMICAL REACTIONS. � Chemical reactions change substances into different ones by breaking and forming chemical bonds. � Reactants are changed during a chemical reaction � Products are made by a chemical reaction.

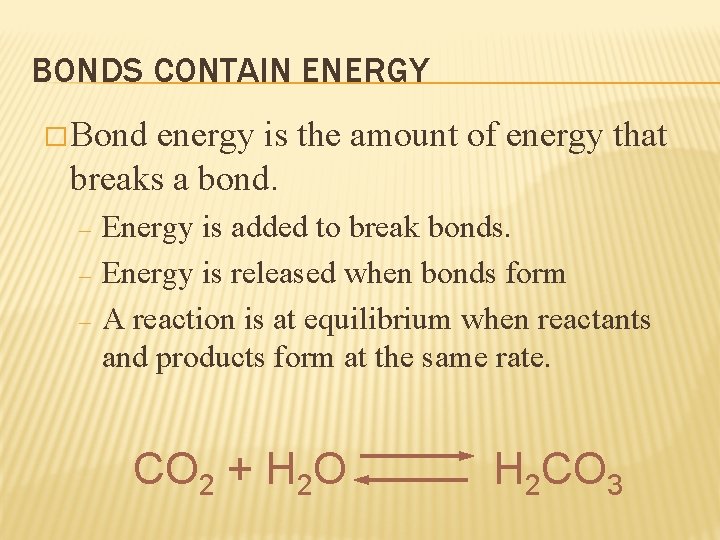
BONDS CONTAIN ENERGY � Bond energy is the amount of energy that breaks a bond. – – – Energy is added to break bonds. Energy is released when bonds form A reaction is at equilibrium when reactants and products form at the same rate. CO 2 + H 2 O H 2 CO 3

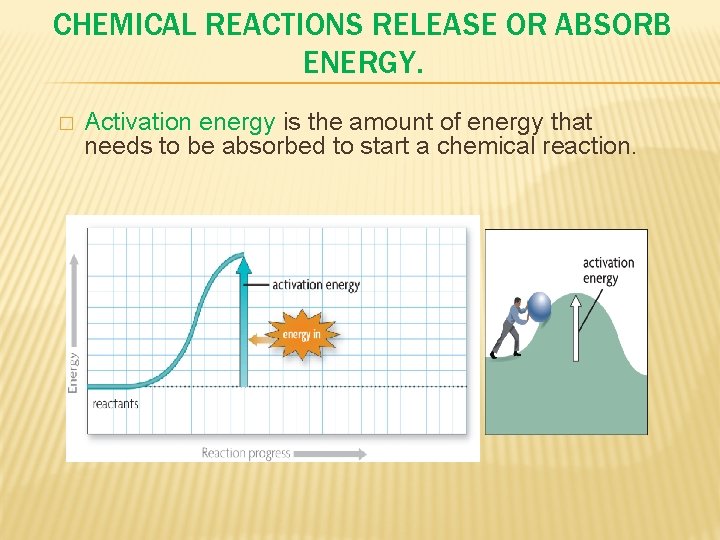
CHEMICAL REACTIONS RELEASE OR ABSORB ENERGY. � Activation energy is the amount of energy that needs to be absorbed to start a chemical reaction.

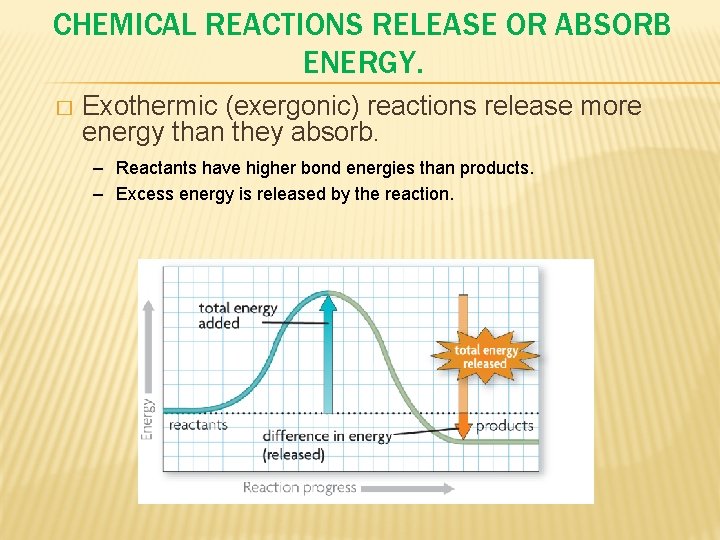
CHEMICAL REACTIONS RELEASE OR ABSORB ENERGY. � Exothermic (exergonic) reactions release more energy than they absorb. – Reactants have higher bond energies than products. – Excess energy is released by the reaction.

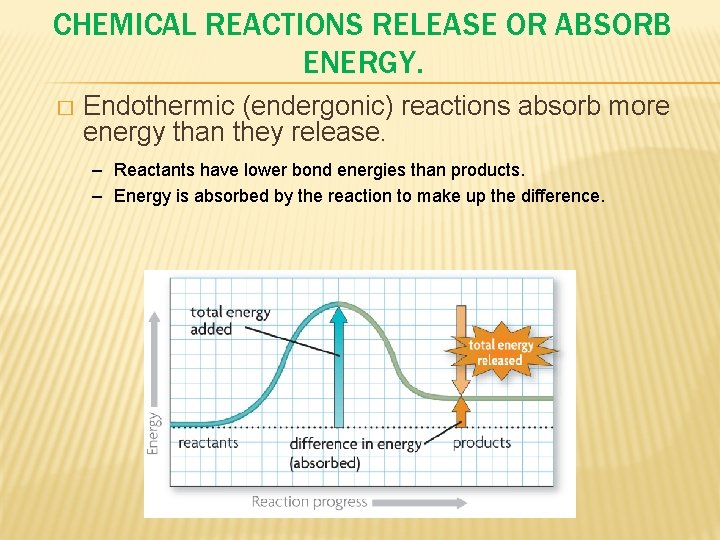
CHEMICAL REACTIONS RELEASE OR ABSORB ENERGY. � Endothermic (endergonic) reactions absorb more energy than they release. – Reactants have lower bond energies than products. – Energy is absorbed by the reaction to make up the difference.

PRACTICE � Think of 7 -10 chemical reaction and determine if they are exothermic or endothermic � Draw an energy diagram for a sprinter

HOMEWORK �Complete Chapter 2 Review numbers 1 -9 (support why you picked each answer)

APPLICATION � Draw an energy diagram for each reaction you determined in the Practice

CLOSING All life processes require energy
 “a sound mind is in a sound body”
“a sound mind is in a sound body” What is sound
What is sound Introduction to sound
Introduction to sound Sound energy travels on a wave
Sound energy travels on a wave Spund energy
Spund energy Puget sound energy investor relations
Puget sound energy investor relations What is enjambment in poetry
What is enjambment in poetry Heat light and sound energy
Heat light and sound energy What is sound energy grade 4
What is sound energy grade 4 Sound energy definition
Sound energy definition Sound is a form of
Sound is a form of Sound energy
Sound energy Work and energy
Work and energy Sound energy
Sound energy Sankey diagram for one bounce of a ball answers
Sankey diagram for one bounce of a ball answers