Introduction to Physical Science Monday Wednesday Thursday Tom



































- Slides: 38

Introduction to Physical Science Monday, Wednesday, Thursday Tom Burbine tomburbine@astro. umass. edu

• Fission – Splitting of the nucleus of a heavy atom with the release of energy • Fusion – Combination of the nuclei of light atoms to form heavier nuclei with the release of energy

Energy Source for Sun • Fusing hydrogen into helium – Hydrogen nucleus – 1 proton – Helium nucleus – 2 protons, 2 neutrons • Need high temperatures for this to occur • ~10 to 14 million degrees Kelvin

http: //www. astronomynotes. com/starsun/s 3. htm

http: //www. astronomynotes. com/starsun/s 3. htm


How does Fusion Convert Mass to Energy • What is the most famous formula in the world?

E = mc 2 • • m is mass in kilograms c is speed of light in meters/s E (energy) is in joules very small amounts of mass may be converted into a very large amount of energy

Who came up with it?

http: //msnbcmedia 3. msn. com/j/msnbc/Components/Photos/z_Projects_in_progress/050418_Einstein/050405_einstein_tongue. widec. jpg

Law • Law of Conservation of mass and energy – Sum of all mass and energy (converted into the same units) must always remain constant during any physical process


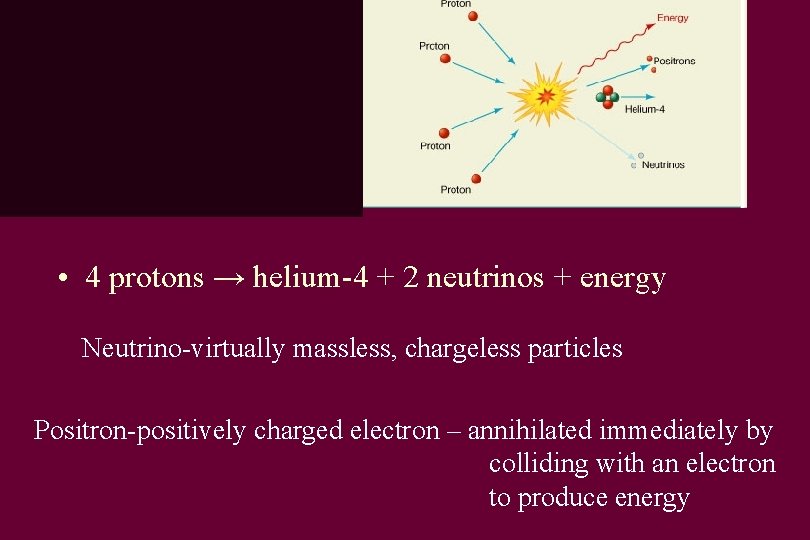
Reaction • 4 protons → helium-4 + 2 neutrinos + energy Neutrino-virtually massless, chargeless particles Positron-positively charged electron – annihilated immediately by colliding with an electron to produce energy

Antiparticles • Antiparticle – particle with the same mass and opposite electric charge • Antiparticles make up antimatter • Annihilation – when a particle and an antiparticle collide • Antimatter is said to be the most costly substance in existence, with an estimated cost of $62. 5 trillion per milligram.

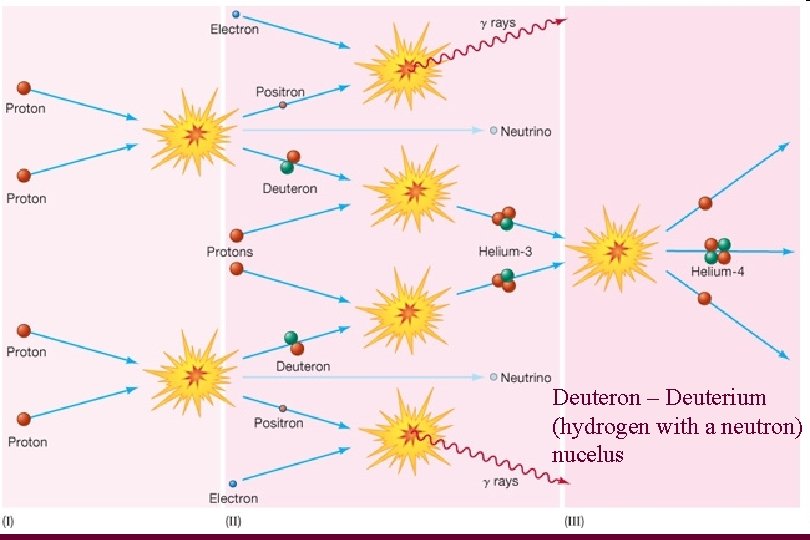
Fusion reaction • Much more complicated than 4 protons → helium-4 + 2 neutrinos + energy


Deuteron – Deuterium (hydrogen with a neutron) nucelus

Proton-Proton Chain Reaction • This reaction occurs ~1038 times each second • It if occurred faster, Sun would run out of fuel

Neutrinos • Neutrinos – almost massless particles • No charge • It takes a neutrino about 2 seconds to exit the Sun

Fusion • The rate of nuclear fusion is a function of temperature • Hotter temperature – higher fusion rate • Lower temperature – lower fusion rate • If the Sun gets hotter or colder, it may not be good for life on Earth

What is happening to the amount of Helium in the Sun? • A) Its increasing • B) its decreasing • C) Its staying the same

What is happening to the amount of Helium in the Sun? • A) Its increasing • B) its decreasing • C) Its staying the same

Valence Electrons • Electrons in the outermost occupied shell of an atom • Forms chemical bonds


Ion • Ion – an electrically charged particle created when an atom either loses or gains one or more electrons




Ionic Bond • Ionic bond – chemical bond in which an attractive electric force holds an opposite charge together

Covalent Bond • Covalent bond – a chemical bond in which atoms are held by their mutual attraction for two or more electrons share • Covalent bonds form molecules



Metallic Bonds • A chemical bond in which positively charged metal ions are held together within a “fluid” • Electromagnetic interaction between conduction electrons and gathered in an "electron sea“ • Atoms "share" electrons that float about in a general electron cloud


Charges need to balance • Na ions carry a +1 charge • Cl ions carry a -1 charge • Na+ + Cl- → Na. Cl (Sodium chloride)

• HCl – Hydrogen Chloride • Li 2 O – Lithium Oxide • CO – Carbon Monoxide • CO 2 – Carbon Dioxide


Any Questions?
 Monday tuesday wednesday thursday friday calendar
Monday tuesday wednesday thursday friday calendar Monday tuesday wednesday thursday friday saturday sunday
Monday tuesday wednesday thursday friday saturday sunday Monday tuesday is my weekend
Monday tuesday is my weekend Sunday monday tuesday wednesday
Sunday monday tuesday wednesday Marvelous monday terrific tuesday wonderful wednesday
Marvelous monday terrific tuesday wonderful wednesday Enum day sunday=1 monday tuesday=5
Enum day sunday=1 monday tuesday=5 Monday=621 tuesday=732 wednesday=933
Monday=621 tuesday=732 wednesday=933 Monday=621 tuesday=732 wednesday=933
Monday=621 tuesday=732 wednesday=933 Why did the yazoo land fraud occur?
Why did the yazoo land fraud occur? Tomtom go 910 update
Tomtom go 910 update What does tom symbolize in the devil and tom walker
What does tom symbolize in the devil and tom walker 3 branches of science
3 branches of science Natural science vs physical science
Natural science vs physical science My favorite subject is biology
My favorite subject is biology Science fusion digital lessons
Science fusion digital lessons Wednesday evening prayer
Wednesday evening prayer Wednesday seminar
Wednesday seminar Ib history ia grade boundaries
Ib history ia grade boundaries Web analytics wednesday
Web analytics wednesday Wednesday diary
Wednesday diary Happy wednesday february
Happy wednesday february My favourite day is monday
My favourite day is monday Skinny wednesday
Skinny wednesday Pat winlink
Pat winlink Happy wednesday
Happy wednesday Happy wednesday march
Happy wednesday march English class is wednesday
English class is wednesday Wednesday journal prompts
Wednesday journal prompts Tuesday bell work
Tuesday bell work Wednesday bellringer
Wednesday bellringer Wednesday bellwork
Wednesday bellwork Wednesday good morning
Wednesday good morning Thursday prayer
Thursday prayer Wednesday bell ringer
Wednesday bell ringer Wednesday syllables
Wednesday syllables Wednesday lunch
Wednesday lunch Response to happy monday
Response to happy monday Happy wednesday
Happy wednesday Thursday prayer
Thursday prayer