Introduction to Fluid Mechanics Fluid Mechanics is concerned




- Slides: 25

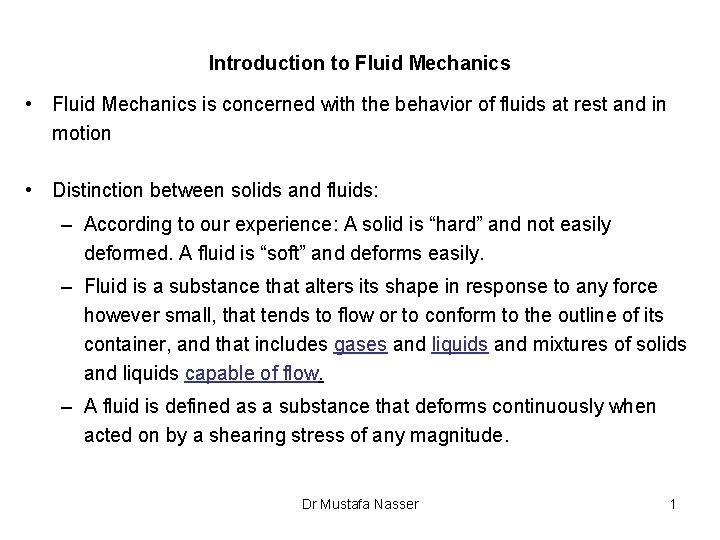
Introduction to Fluid Mechanics • Fluid Mechanics is concerned with the behavior of fluids at rest and in motion • Distinction between solids and fluids: – According to our experience: A solid is “hard” and not easily deformed. A fluid is “soft” and deforms easily. – Fluid is a substance that alters its shape in response to any force however small, that tends to flow or to conform to the outline of its container, and that includes gases and liquids and mixtures of solids and liquids capable of flow. – A fluid is defined as a substance that deforms continuously when acted on by a shearing stress of any magnitude. Dr Mustafa Nasser 1

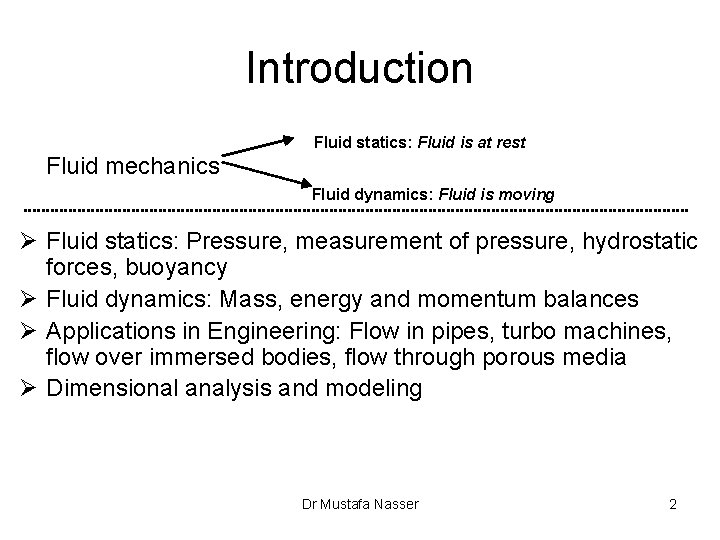
Introduction Fluid statics: Fluid is at rest Fluid mechanics Fluid dynamics: Fluid is moving Ø Fluid statics: Pressure, measurement of pressure, hydrostatic forces, buoyancy Ø Fluid dynamics: Mass, energy and momentum balances Ø Applications in Engineering: Flow in pipes, turbo machines, flow over immersed bodies, flow through porous media Ø Dimensional analysis and modeling Dr Mustafa Nasser 2

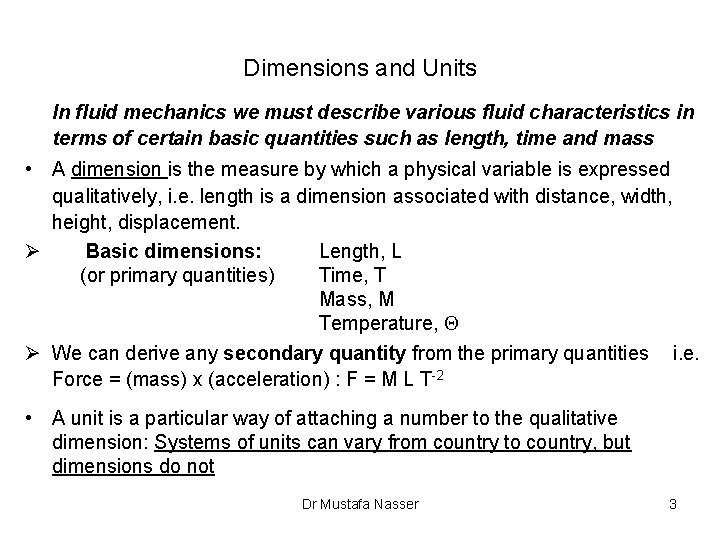
Dimensions and Units In fluid mechanics we must describe various fluid characteristics in terms of certain basic quantities such as length, time and mass • A dimension is the measure by which a physical variable is expressed qualitatively, i. e. length is a dimension associated with distance, width, height, displacement. Ø Basic dimensions: Length, L (or primary quantities) Time, T Mass, M Temperature, Q Ø We can derive any secondary quantity from the primary quantities i. e. Force = (mass) x (acceleration) : F = M L T-2 • A unit is a particular way of attaching a number to the qualitative dimension: Systems of units can vary from country to country, but dimensions do not Dr Mustafa Nasser 3

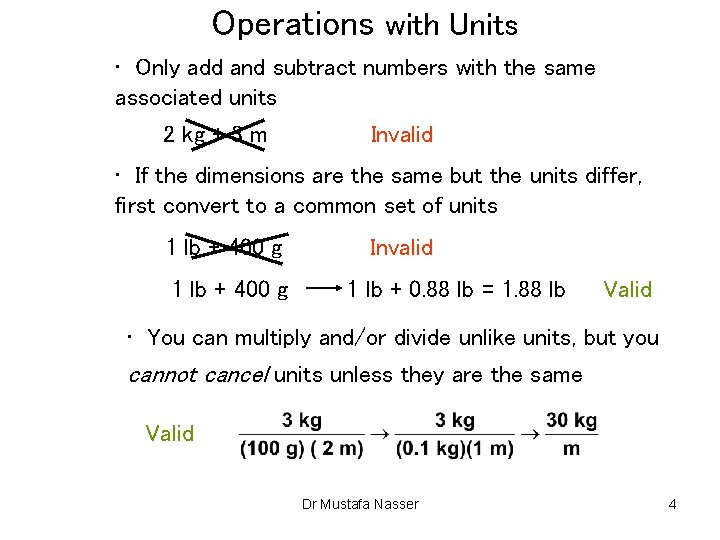
Operations with Units • Only add and subtract numbers with the same associated units 2 kg + 3 m Invalid • If the dimensions are the same but the units differ, first convert to a common set of units 1 lb + 400 g Invalid 1 lb + 0. 88 lb = 1. 88 lb Valid • You can multiply and/or divide unlike units, but you cannot cancel units unless they are the same Valid Dr Mustafa Nasser 4

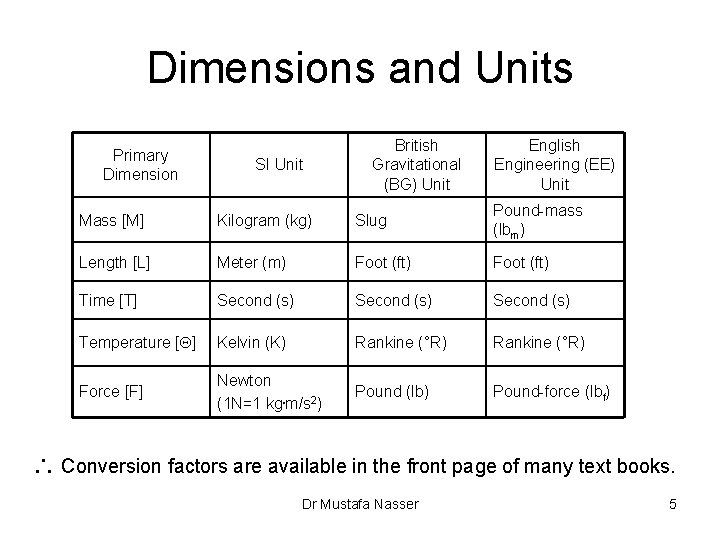
Dimensions and Units Primary Dimension SI Unit British Gravitational (BG) Unit English Engineering (EE) Unit Mass [M] Kilogram (kg) Slug Pound-mass (lbm) Length [L] Meter (m) Foot (ft) Time [T] Second (s) Temperature [Q] Kelvin (K) Rankine (°R) Force [F] Newton (1 N=1 kg. m/s 2) Pound (lb) Pound-force (lbf) Conversion factors are available in the front page of many text books. Dr Mustafa Nasser 5

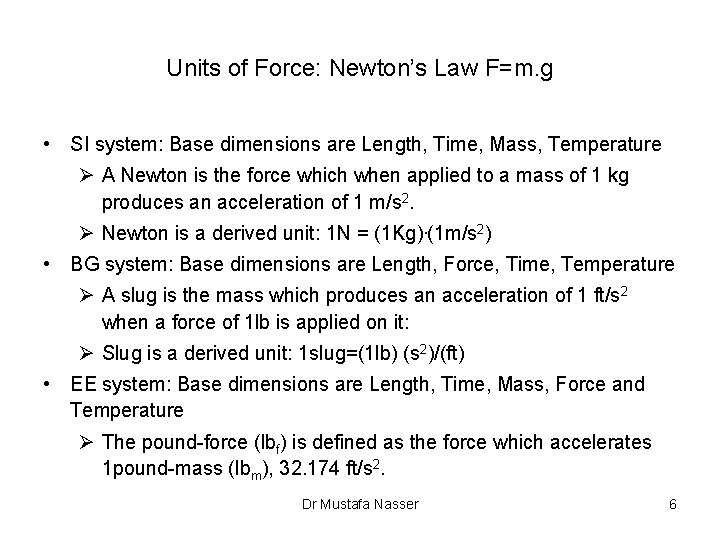
Units of Force: Newton’s Law F=m. g • SI system: Base dimensions are Length, Time, Mass, Temperature Ø A Newton is the force which when applied to a mass of 1 kg produces an acceleration of 1 m/s 2. Ø Newton is a derived unit: 1 N = (1 Kg). (1 m/s 2) • BG system: Base dimensions are Length, Force, Time, Temperature Ø A slug is the mass which produces an acceleration of 1 ft/s 2 when a force of 1 lb is applied on it: Ø Slug is a derived unit: 1 slug=(1 lb) (s 2)/(ft) • EE system: Base dimensions are Length, Time, Mass, Force and Temperature Ø The pound-force (lbf) is defined as the force which accelerates 1 pound-mass (lbm), 32. 174 ft/s 2. Dr Mustafa Nasser 6

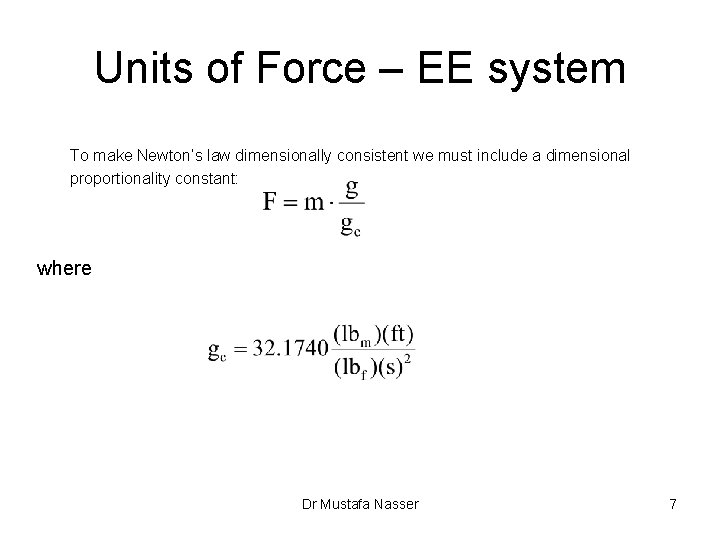
Units of Force – EE system To make Newton’s law dimensionally consistent we must include a dimensional proportionality constant: where Dr Mustafa Nasser 7

Dimensional Homogeneity • All theoretically derived equations are dimensionally homogeneous: dimensions of the left side of the equation must be the same as those on the right side. – Some empirical formulas used in engineering practice are not dimensionally homogeneous • All equations must use consistent units: each term must have the same units. Answers will be incorrect if the units in the equation are not consistent. Always chose the system of units prior to solving the problem Dr Mustafa Nasser 8

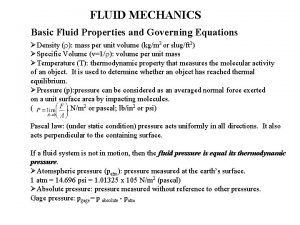
Properties of Fluids Ø Fundamental approach: Study the behavior of individual molecules when trying to describe the behavior of fluids Ø Engineering approach: Characterization of the behavior by considering the average, or macroscopic, value of the quantity of interest, where the average is evaluated over a small volume containing a large number of molecules Treat the fluid as a CONTINUUM: Assume that all the fluid characteristics vary continuously throughout the fluid Dr Mustafa Nasser 9

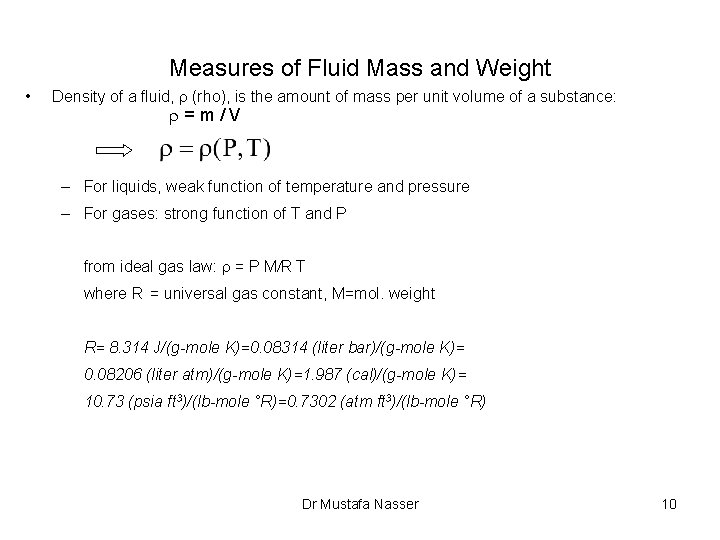
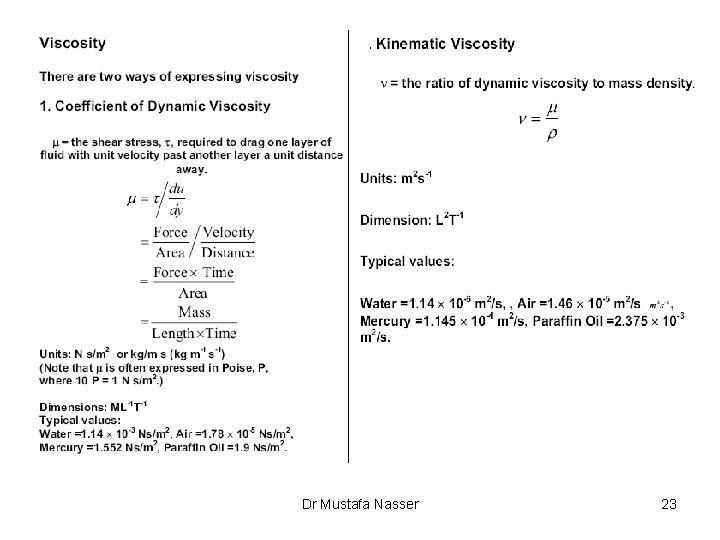
Measures of Fluid Mass and Weight • Density of a fluid, r (rho), is the amount of mass per unit volume of a substance: r=m/V – For liquids, weak function of temperature and pressure – For gases: strong function of T and P from ideal gas law: r = P M/R T where R = universal gas constant, M=mol. weight R= 8. 314 J/(g-mole K)=0. 08314 (liter bar)/(g-mole K)= 0. 08206 (liter atm)/(g-mole K)=1. 987 (cal)/(g-mole K)= 10. 73 (psia ft 3)/(lb-mole °R)=0. 7302 (atm ft 3)/(lb-mole °R) Dr Mustafa Nasser 10

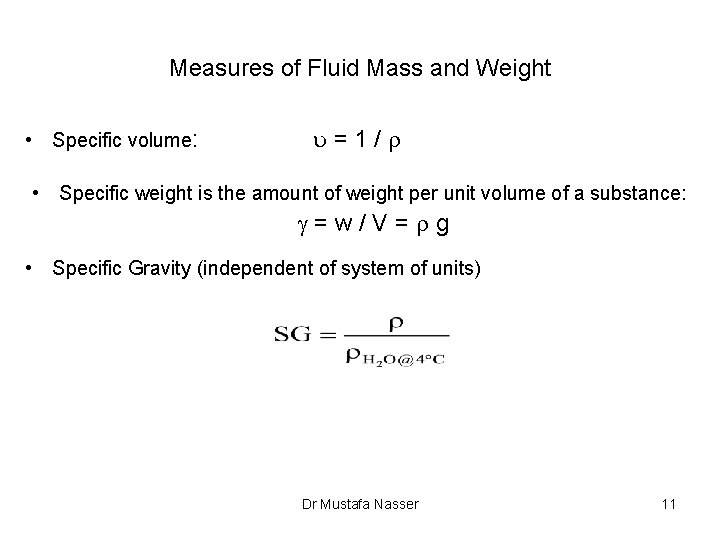
Measures of Fluid Mass and Weight • Specific volume: u=1/r • Specific weight is the amount of weight per unit volume of a substance: g=w/V=rg • Specific Gravity (independent of system of units) Dr Mustafa Nasser 11

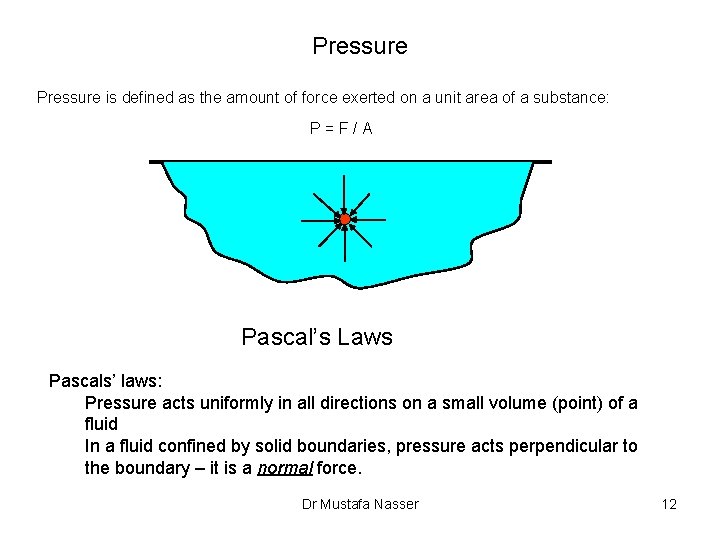
Pressure is defined as the amount of force exerted on a unit area of a substance: P=F/A Pascal’s Laws Pascals’ laws: Pressure acts uniformly in all directions on a small volume (point) of a fluid In a fluid confined by solid boundaries, pressure acts perpendicular to the boundary – it is a normal force. Dr Mustafa Nasser 12

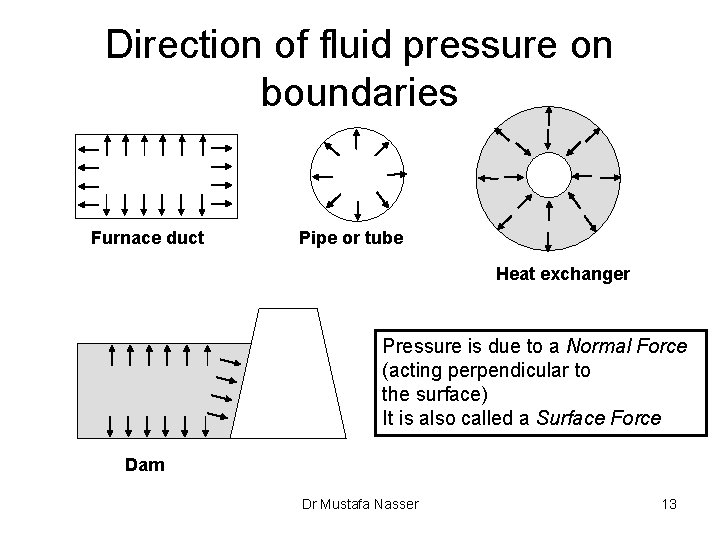
Direction of fluid pressure on boundaries Furnace duct Pipe or tube Heat exchanger Pressure is due to a Normal Force (acting perpendicular to the surface) It is also called a Surface Force Dam Dr Mustafa Nasser 13

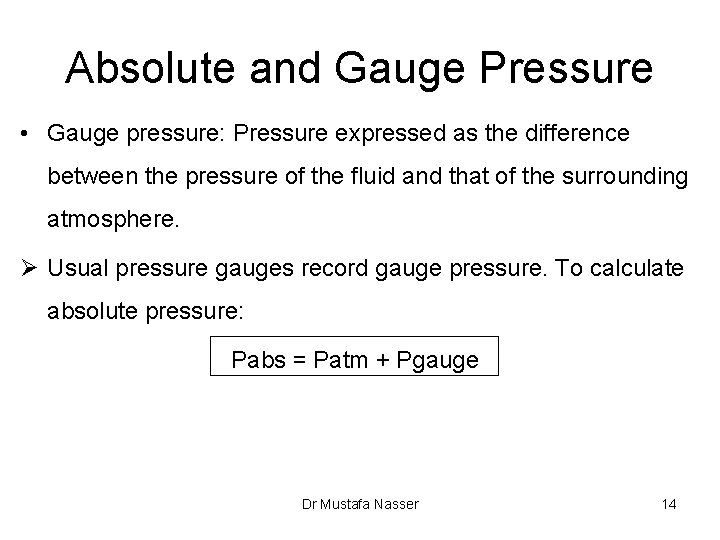
Absolute and Gauge Pressure • Gauge pressure: Pressure expressed as the difference between the pressure of the fluid and that of the surrounding atmosphere. Ø Usual pressure gauges record gauge pressure. To calculate absolute pressure: Pabs = Patm + Pgauge Dr Mustafa Nasser 14

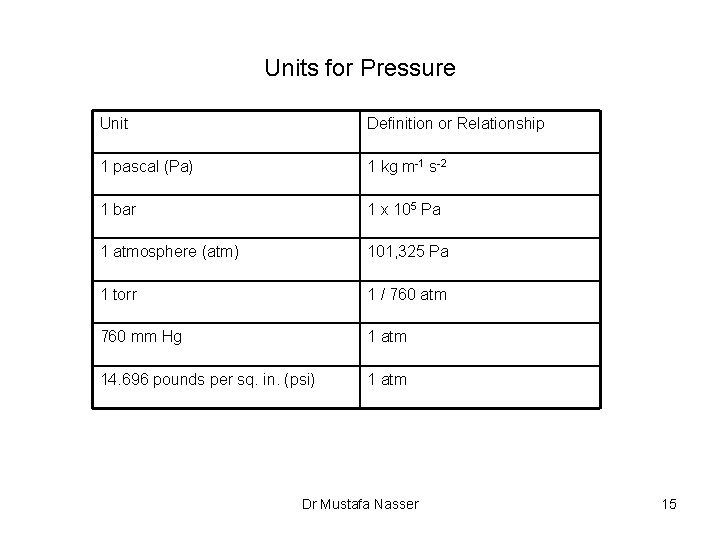
Units for Pressure Unit Definition or Relationship 1 pascal (Pa) 1 kg m-1 s-2 1 bar 1 x 105 Pa 1 atmosphere (atm) 101, 325 Pa 1 torr 1 / 760 atm 760 mm Hg 1 atm 14. 696 pounds per sq. in. (psi) 1 atm Dr Mustafa Nasser 15

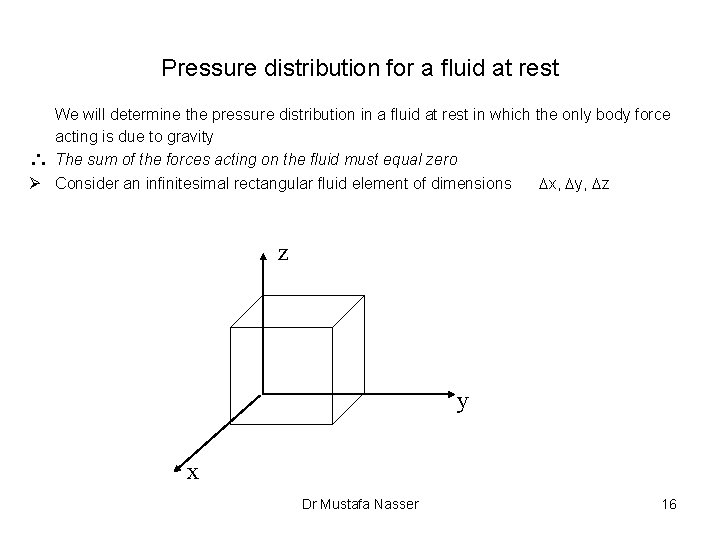
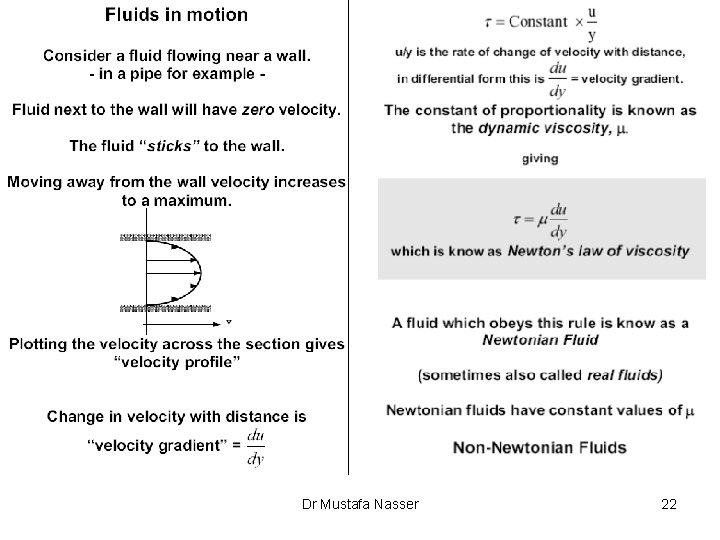
Pressure distribution for a fluid at rest We will determine the pressure distribution in a fluid at rest in which the only body force acting is due to gravity The sum of the forces acting on the fluid must equal zero Ø Consider an infinitesimal rectangular fluid element of dimensions Dx, Dy, Dz z y x Dr Mustafa Nasser 16

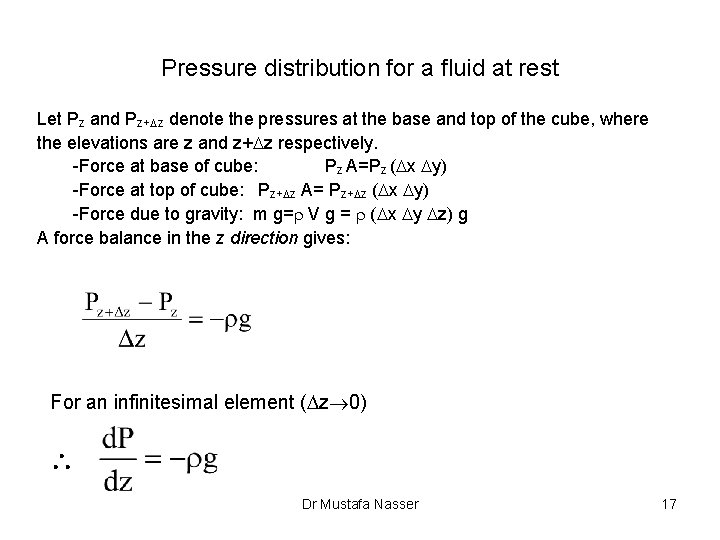
Pressure distribution for a fluid at rest Let Pz and Pz+Dz denote the pressures at the base and top of the cube, where the elevations are z and z+Dz respectively. -Force at base of cube: Pz A=Pz (Dx Dy) -Force at top of cube: Pz+Dz A= Pz+Dz (Dx Dy) -Force due to gravity: m g=r V g = r (Dx Dy Dz) g A force balance in the z direction gives: For an infinitesimal element (Dz 0) Dr Mustafa Nasser 17

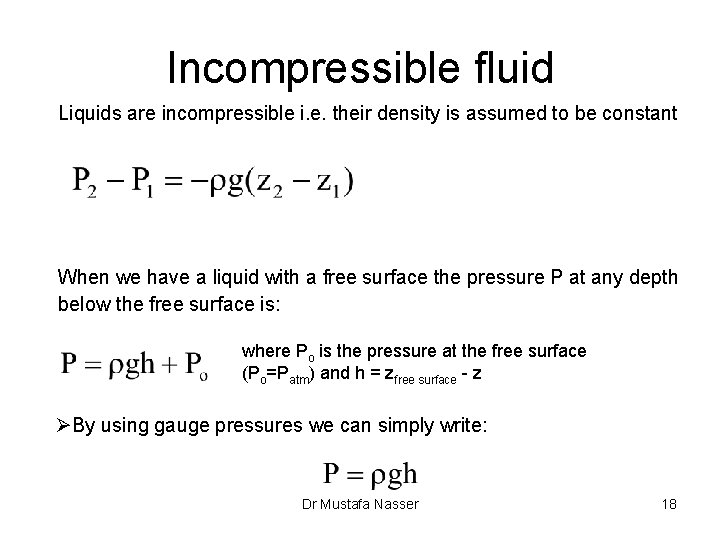
Incompressible fluid Liquids are incompressible i. e. their density is assumed to be constant When we have a liquid with a free surface the pressure P at any depth below the free surface is: where Po is the pressure at the free surface (Po=Patm) and h = zfree surface - z ØBy using gauge pressures we can simply write: Dr Mustafa Nasser 18

Examples SG= 13. 6 Dr Mustafa Nasser 19

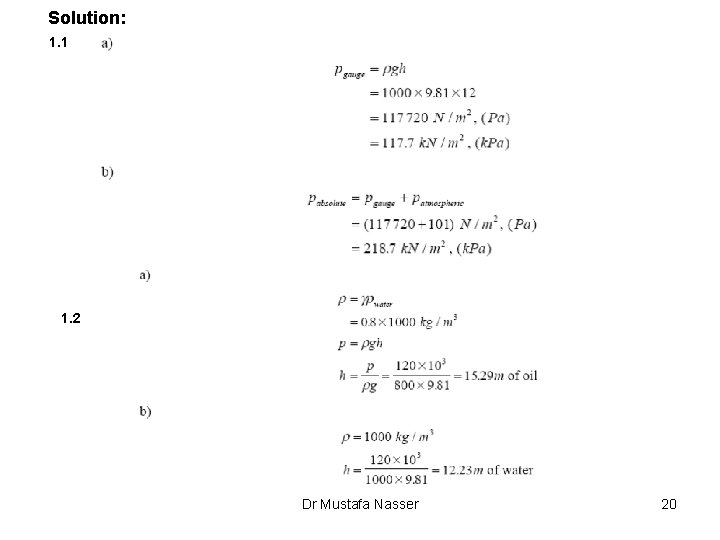
Solution: 1. 1 1. 2 Dr Mustafa Nasser 20

1. 3 Dr Mustafa Nasser 21

Dr Mustafa Nasser 22

Dr Mustafa Nasser 23

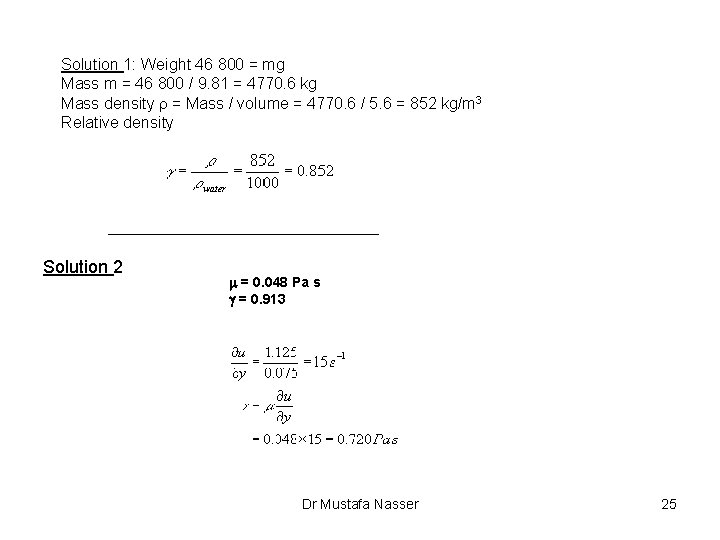
Example 1: 5. 6 m 3 of oil weighs 46 800 N. Find its mass density, and relative density, g. Example 2: In a fluid the velocity measured at a distance of 75 mm from the boundary is 1. 125 m/s. The fluid has absolute viscosity 0. 048 Pa s and relative density 0. 913. What is the velocity gradient and shear stress at the boundary assuming a linear velocity distribution. Dr Mustafa Nasser 24

Solution 1: Weight 46 800 = mg Mass m = 46 800 / 9. 81 = 4770. 6 kg Mass density r = Mass / volume = 4770. 6 / 5. 6 = 852 kg/m 3 Relative density Solution 2 m = 0. 048 Pa s g = 0. 913 Dr Mustafa Nasser 25
 Fluid kinematics
Fluid kinematics Synovial fluid
Synovial fluid P1-p2
P1-p2 Fluid statics deals with fluid at rest
Fluid statics deals with fluid at rest Transcellular fluid
Transcellular fluid Interstitial fluid vs extracellular fluid
Interstitial fluid vs extracellular fluid Hypoosmotic
Hypoosmotic Movement of body fluids
Movement of body fluids Fluid thrill positive
Fluid thrill positive Stokes fluid mechanics
Stokes fluid mechanics Fluid mechanics subject code
Fluid mechanics subject code Fluid mechanics subject code
Fluid mechanics subject code Dimensional analysis fluid mechanics
Dimensional analysis fluid mechanics Friction factor for laminar flow
Friction factor for laminar flow Fluid mechanics pdhpe
Fluid mechanics pdhpe Non dimensional numbers
Non dimensional numbers Dimensionless groups in fluid mechanics
Dimensionless groups in fluid mechanics Fluid mechanics energy equation
Fluid mechanics energy equation Critical flow examples
Critical flow examples Critical reynolds number
Critical reynolds number Hydrostatic force on a vertical wall formula
Hydrostatic force on a vertical wall formula Chapter 8 fluid mechanics
Chapter 8 fluid mechanics Similitude fluid mechanics
Similitude fluid mechanics Momentum equation fluid mechanics examples
Momentum equation fluid mechanics examples Dynamic viscosity units
Dynamic viscosity units Euler equation in fluid mechanics
Euler equation in fluid mechanics