http chemconnections orggeneralchem 108MagnesiumZincwo 1 mov Experimentally Determining

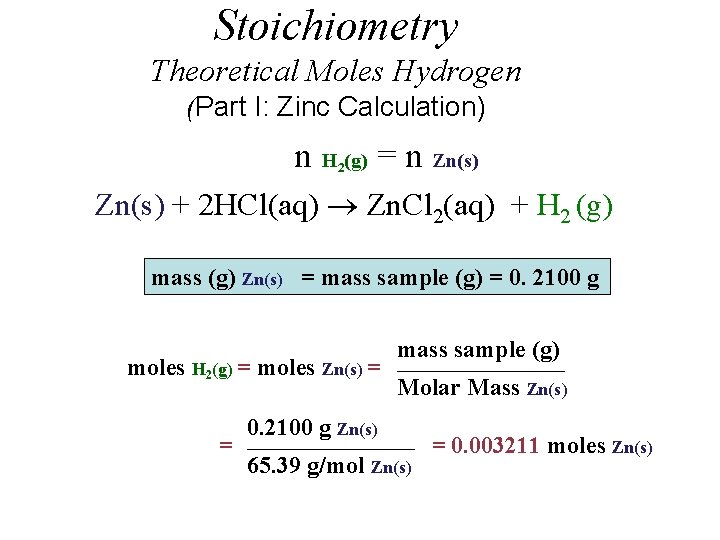
- Slides: 19

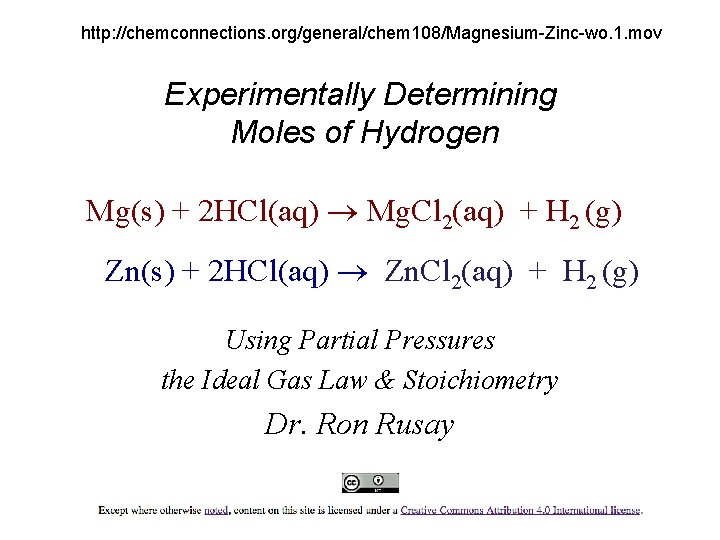
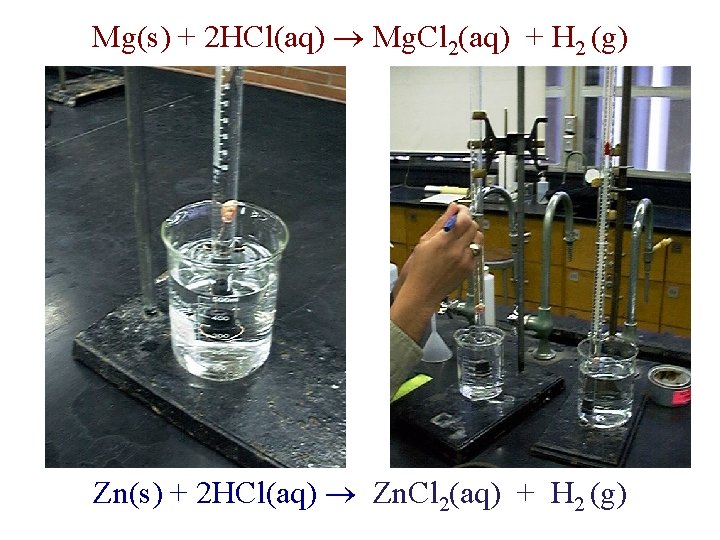
http: //chemconnections. org/general/chem 108/Magnesium-Zinc-wo. 1. mov Experimentally Determining Moles of Hydrogen Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 (g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) Using Partial Pressures the Ideal Gas Law & Stoichiometry Dr. Ron Rusay


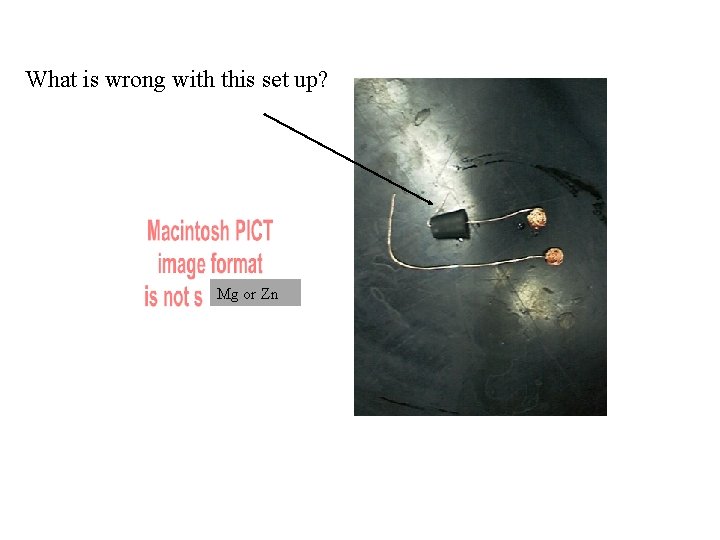
What is wrong with this set up? Mg or Zn

Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 (g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g)

Mg or Zn

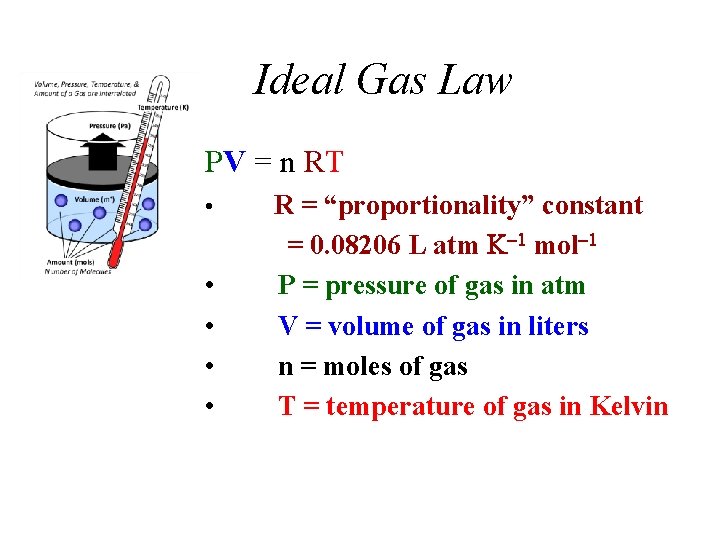
Ideal Gas Law PV = n RT • • • R = “proportionality” constant = 0. 08206 L atm mol P = pressure of gas in atm V = volume of gas in liters n = moles of gas T = temperature of gas in Kelvin

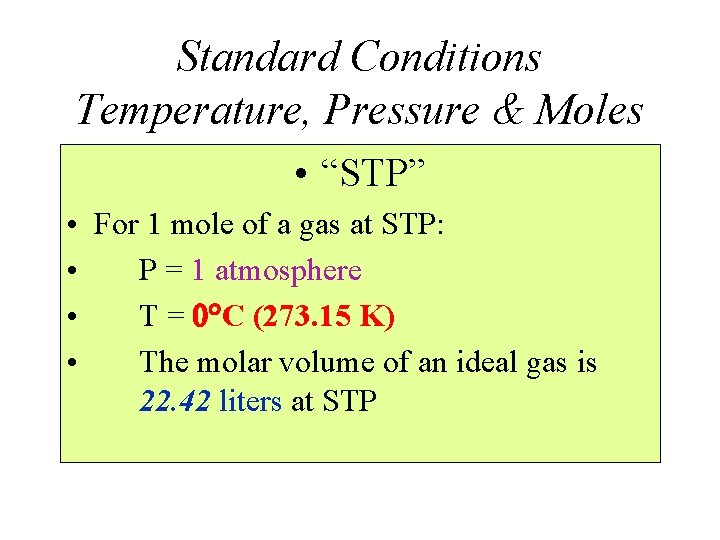
Standard Conditions Temperature, Pressure & Moles • “STP” • For 1 mole of a gas at STP: • P = 1 atmosphere • T = C (273. 15 K) • The molar volume of an ideal gas is 22. 42 liters at STP

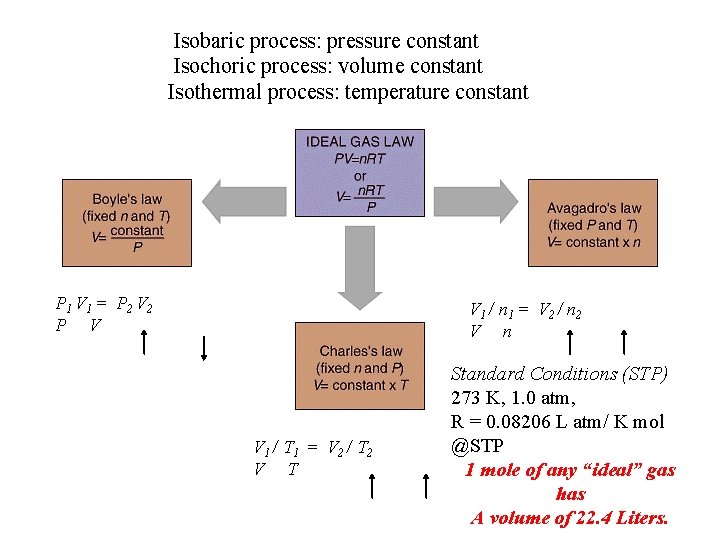
Isobaric process: pressure constant Isochoric process: volume constant Isothermal process: temperature constant P 1 V 1 = P 2 V 2 P V V 1 / n 1 = V 2 / n 2 V n V 1 / T 1 = V 2 / T 2 V T Standard Conditions (STP) 273 K, 1. 0 atm, R = 0. 08206 L atm/ K mol @STP 1 mole of any “ideal” gas has A volume of 22. 4 Liters.

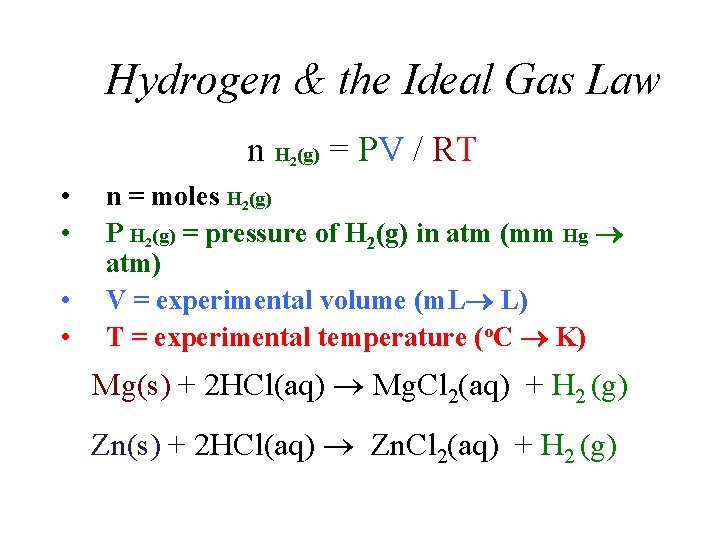
Hydrogen & the Ideal Gas Law n H (g) = PV / RT 2 • • n = moles H 2(g) P H 2(g) = pressure of H 2(g) in atm (mm Hg atm) V = experimental volume (m. L L) T = experimental temperature (o. C K) Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 (g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g)

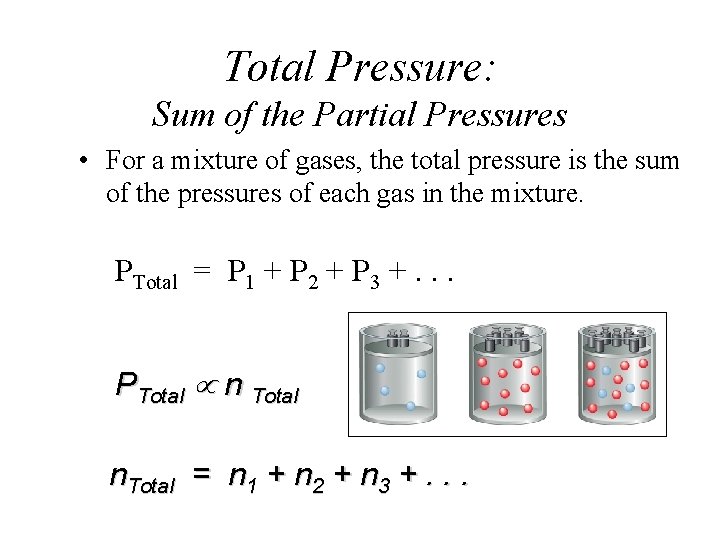
Total Pressure: Sum of the Partial Pressures • For a mixture of gases, the total pressure is the sum of the pressures of each gas in the mixture. PTotal = P 1 + P 2 + P 3 +. . . PTotal n Total n. Total = n 1 + n 2 + n 3 +. . .

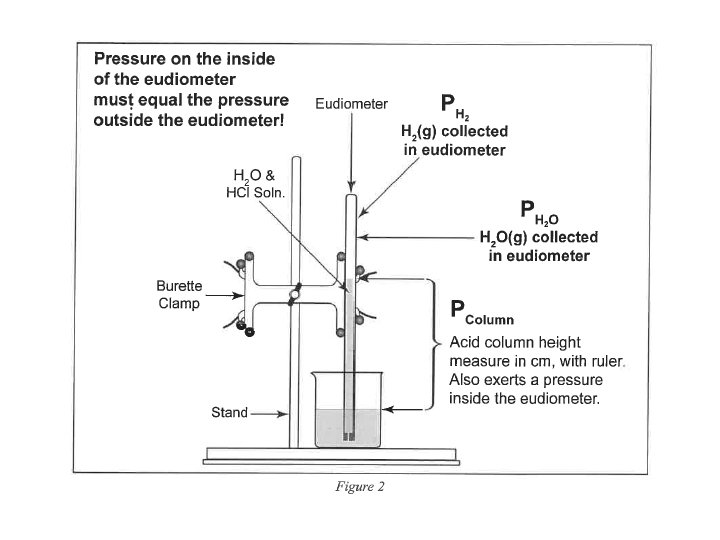
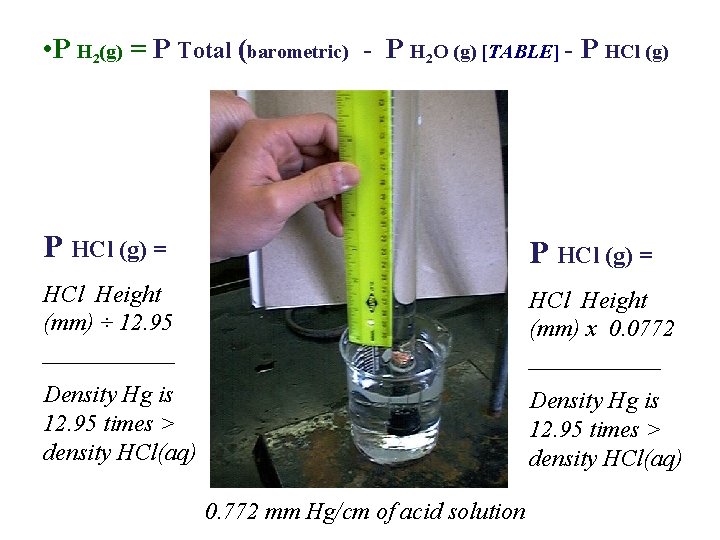
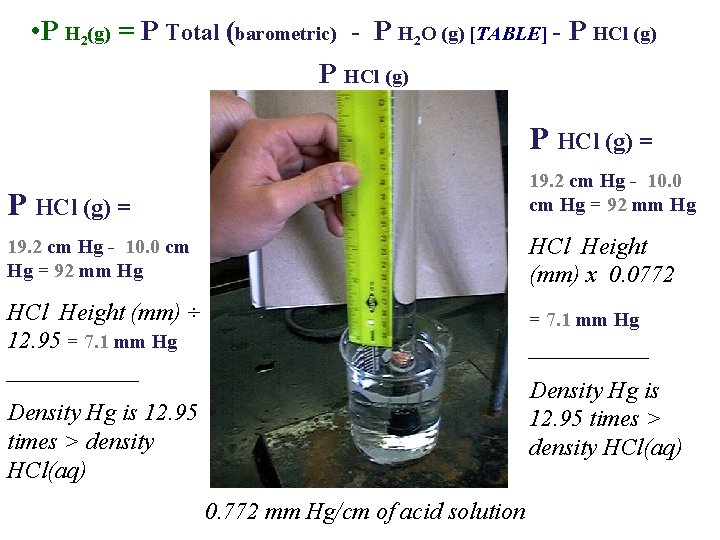
• P H 2(g) = P Total (barometric) - P H 2 O (g) [TABLE] - P HCl (g) = HCl Height (mm) ÷ 12. 95 ______ HCl Height (mm) x 0. 0772 ______ Density Hg is 12. 95 times > density HCl(aq) 0. 772 mm Hg/cm of acid solution

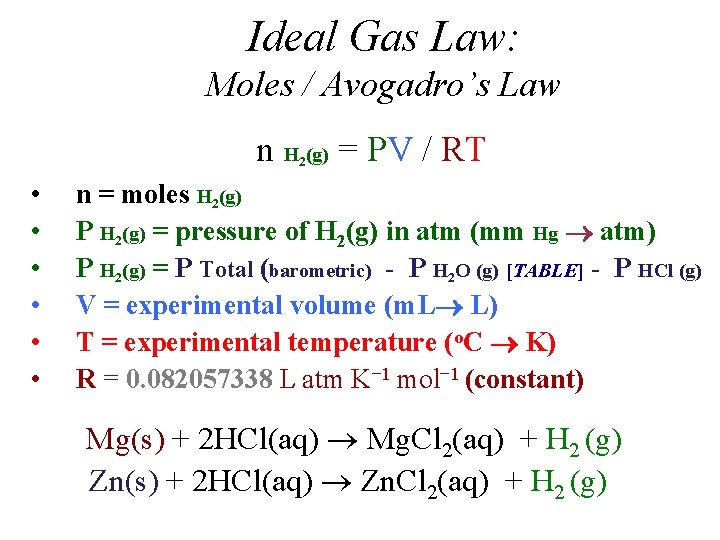
Ideal Gas Law: Moles / Avogadro’s Law n H (g) = PV / RT 2 • • • n = moles H 2(g) P H 2(g) = pressure of H 2(g) in atm (mm Hg atm) P H 2(g) = P Total (barometric) - P H 2 O (g) [TABLE] - P HCl (g) V = experimental volume (m. L L) T = experimental temperature (o. C K) R = 0. 082057338 L atm K− 1 mol− 1 (constant) Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 (g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g)
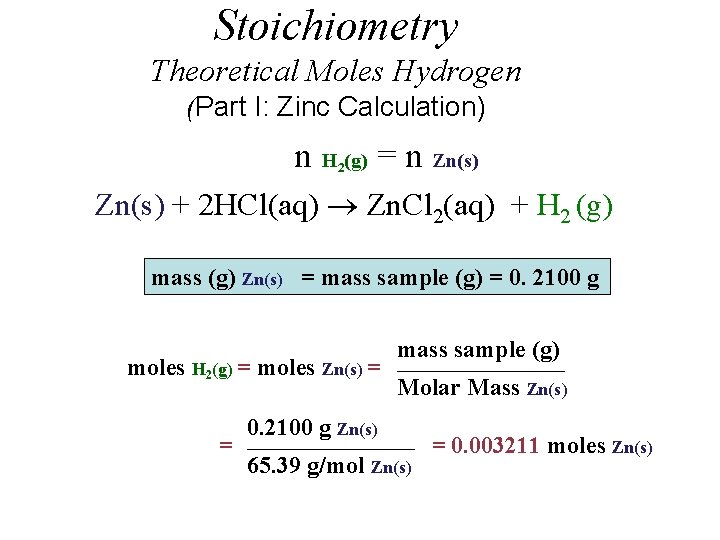
Stoichiometry Theoretical Moles Hydrogen (Part I: Zinc Calculation) n H (g) = n Zn(s) 2 Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) mass (g) Zn(s) = mass sample (g) = 0. 2100 g moles H 2(g) = moles Zn(s) = = mass sample (g) _____________________ Molar Mass Zn(s) 0. 2100 g Zn(s) _____________________ 65. 39 g/mol Zn(s) = 0. 003211 moles Zn(s)

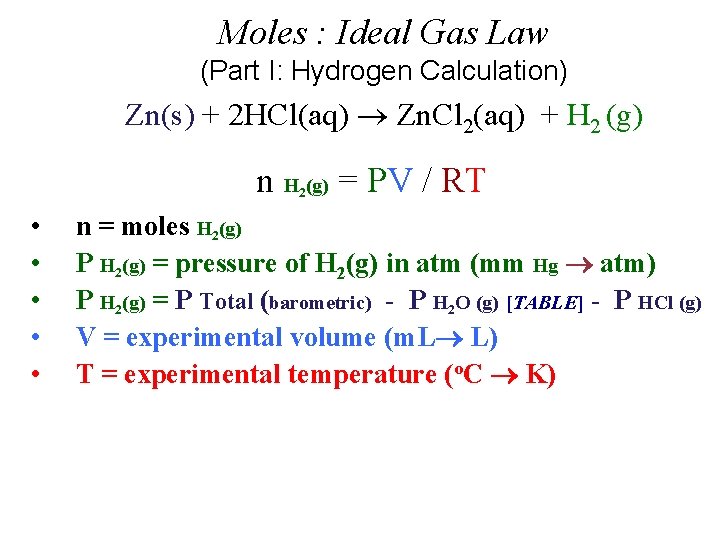
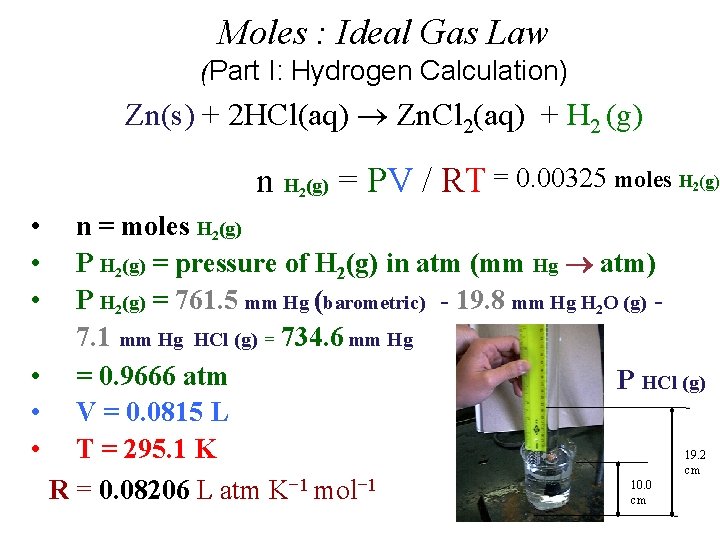
Moles : Ideal Gas Law (Part I: Hydrogen Calculation) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) n H (g) = PV / RT 2 • • • n = moles H 2(g) P H 2(g) = pressure of H 2(g) in atm (mm Hg atm) P H 2(g) = P Total (barometric) - P H 2 O (g) [TABLE] - P HCl (g) V = experimental volume (m. L L) T = experimental temperature (o. C K)

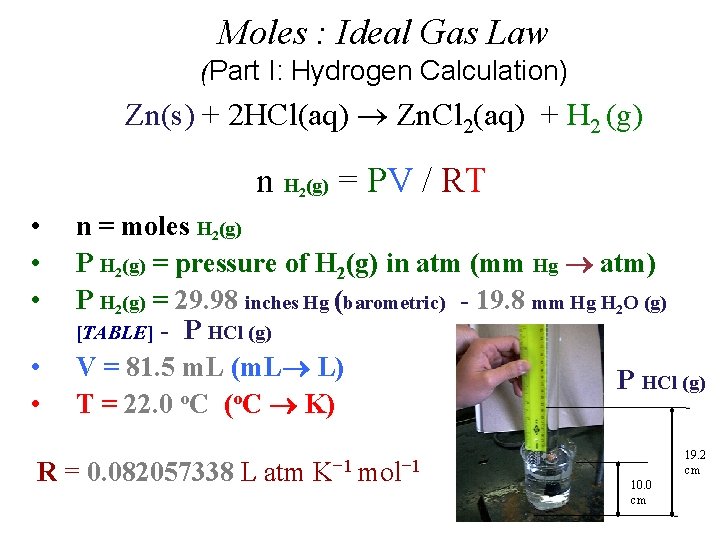
Moles : Ideal Gas Law (Part I: Hydrogen Calculation) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) n H (g) = PV / RT 2 • • • n = moles H 2(g) P H 2(g) = pressure of H 2(g) in atm (mm Hg atm) P H 2(g) = 29. 98 inches Hg (barometric) - 19. 8 mm Hg H 2 O (g) [TABLE] - P HCl (g) V = 81. 5 m. L (m. L L) P HCl (g) T = 22. 0 o. C (o. C K) R = 0. 082057338 L atm K− 1 mol− 1 19. 2 cm 10. 0 cm

• P H 2(g) = P Total (barometric) - P H 2 O (g) [TABLE] - P HCl (g) = 19. 2 cm Hg - 10. 0 cm Hg = 92 mm Hg HCl Height (mm) x 0. 0772 HCl Height (mm) ÷ 12. 95 = 7. 1 mm Hg ______ = 7. 1 mm Hg _____ Density Hg is 12. 95 times > density HCl(aq) 0. 772 mm Hg/cm of acid solution

Moles : Ideal Gas Law (Part I: Hydrogen Calculation) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) n H (g) = PV / RT = 0. 00325 moles H (g) 2 • • • 2 n = moles H 2(g) P H 2(g) = pressure of H 2(g) in atm (mm Hg atm) P H 2(g) = 761. 5 mm Hg (barometric) - 19. 8 mm Hg H 2 O (g) 7. 1 mm Hg HCl (g) = 734. 6 mm Hg • = 0. 9666 atm P HCl (g) • V = 0. 0815 L • T = 295. 1 K 19. 2 cm 10. 0 R = 0. 08206 L atm K− 1 mol− 1 cm

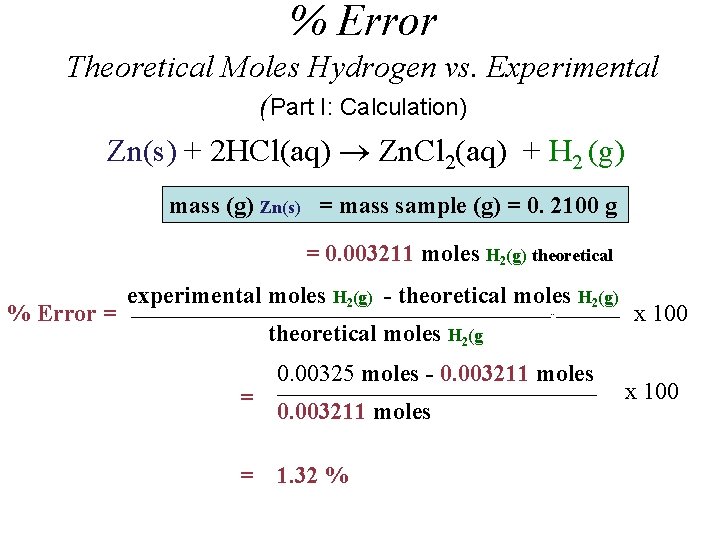
% Error Theoretical Moles Hydrogen vs. Experimental (Part I: Calculation) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2 (g) mass (g) Zn(s) = mass sample (g) = 0. 2100 g = 0. 003211 moles H 2(g) theoretical % Error = experimental moles H 2(g) - theoretical moles H 2(g) _____________________________________________________--________ theoretical moles H 2(g = = 0. 00325 moles - 0. 003211 moles ________________________________________ 0. 003211 moles 1. 32 % x 100

Molar Mass of a Gas (Hydrogen) • PV = n RT • n = g of gas/ MM gas [MM gas = g/mol] • PV = (g of gas/ MM gas)RT • MM gas = g of gas/V (RT/P) Density of gas • MM gas = g of gas/V (RT/P) • MM gas = density of gas (RT/P)
 Congruence of triangles class 9 activity
Congruence of triangles class 9 activity Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com Http //pelatihan tik.ung.ac.id
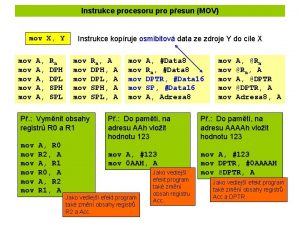
Http //pelatihan tik.ung.ac.id Mov a, 3000 h is legal illegal
Mov a, 3000 h is legal illegal Compiler
Compiler 8051 rr
8051 rr Hex to bcd conversion in 8085
Hex to bcd conversion in 8085 Puls root word definition
Puls root word definition Mov zota
Mov zota Ax-bx
Ax-bx Mov a, #89 h is the example of addressing mode.
Mov a, #89 h is the example of addressing mode. Measurable organizational value template
Measurable organizational value template Mov ah, 9
Mov ah, 9 Data movement instructions in microprocessor
Data movement instructions in microprocessor What is the meaning of the root mob/mov/mot
What is the meaning of the root mob/mov/mot Auma service password
Auma service password Jeu mov
Jeu mov Movzx instruction
Movzx instruction Clr a instruction in 8051
Clr a instruction in 8051 Mov a,b
Mov a,b