Heat and Sound Phases and Phase Changes 1


- Slides: 3

Heat and Sound Phases and Phase Changes 1 Hennig Brand – discovered the first element (phosphorous) Robert Boyle – PV = constant Edmé Mariotte – constant depends on temperature Johann Joachim Becher – Earth separable into vitrifiable, mercurial, combustible (phlogiston) Georg Ernst Stahl – Phlogiston Guillaume Amontons – Absolute zero temperature (when the volume is zero) René Antoine Ferchault de Réaummur – unused temperature system Anders Celcius – Celcius temperature system (0 o = freezing water, 100 o = boiling water) Jaques Alexandre César Charles – PV = constant x (Tcelcius + 273) = constant x Tkelvin) Karl Wilhelm Scheele – Discovered oxygen Joseph Priestley – Learned how to remove oxygen from and add oxygen to air (he called this phlogisticated and dephlogisticated air) Anton Lavoisier – First to create a list of elements William Nicholson – separation of components using electricity John Dalton – Established theory of atoms and measured relative weights for certain elements Sir Humphrey Davy – Used melting and electricity to find more elements Joseph Gay-Lussac – The space occupied by an atom is the same for all elements Count Amedo Avogadro – concept of molecules, Avogadro's number (6. 02 x 1023 atoms in two grams of hydrogen)

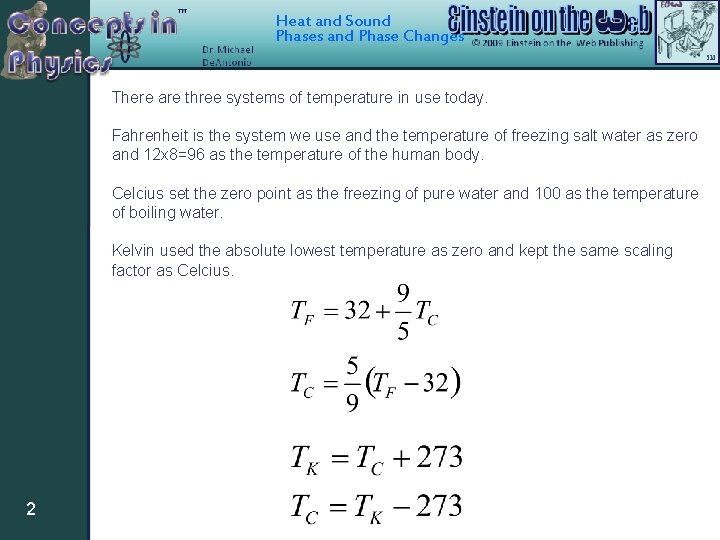
Heat and Sound Phases and Phase Changes There are three systems of temperature in use today. Fahrenheit is the system we use and the temperature of freezing salt water as zero and 12 x 8=96 as the temperature of the human body. Celcius set the zero point as the freezing of pure water and 100 as the temperature of boiling water. Kelvin used the absolute lowest temperature as zero and kept the same scaling factor as Celcius. 2

Heat and Sound Phases and Phase Changes For ideal gases 3 http: //webphysics. ph. msstate. edu/jc/library/12 -4/index. html