Gases Macroscopic Properties of Gases expand to fill

- Slides: 6

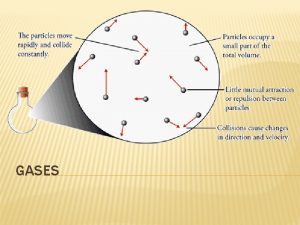
Gases Macroscopic Properties of Gases • • • expand to fill the volume of any container have much lower densities than solids or liquids have highly variable densities, depending on conditions mix with one another readily and thoroughly change volume dramatically with changing temperature General Chemistry CHM 107 Dr Erdal Onurhan Slide 1

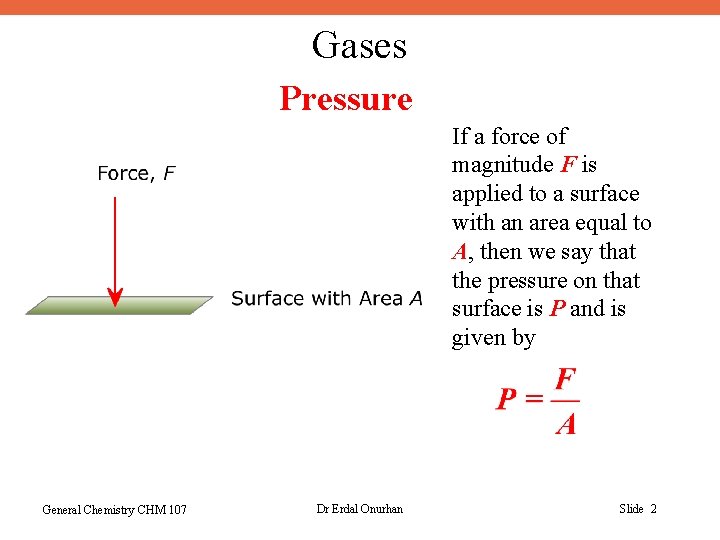
Gases Pressure If a force of magnitude F is applied to a surface with an area equal to A, then we say that the pressure on that surface is P and is given by General Chemistry CHM 107 Dr Erdal Onurhan Slide 2

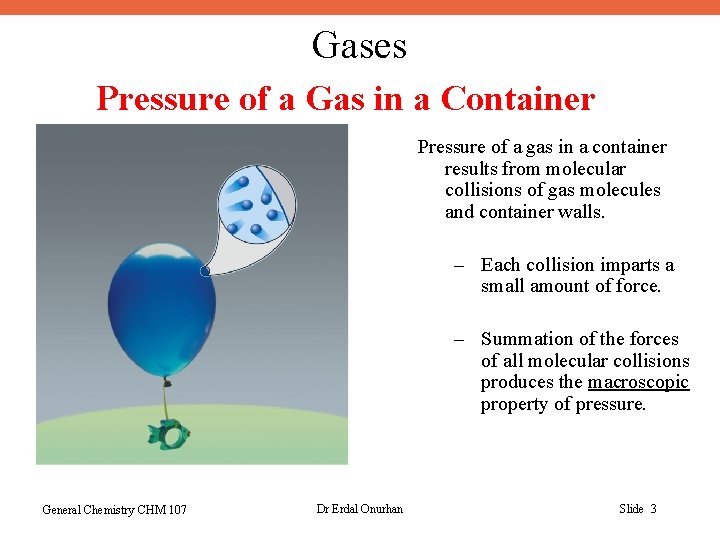
Gases Pressure of a Gas in a Container Pressure of a gas in a container results from molecular collisions of gas molecules and container walls. – Each collision imparts a small amount of force. – Summation of the forces of all molecular collisions produces the macroscopic property of pressure. General Chemistry CHM 107 Dr Erdal Onurhan Slide 3

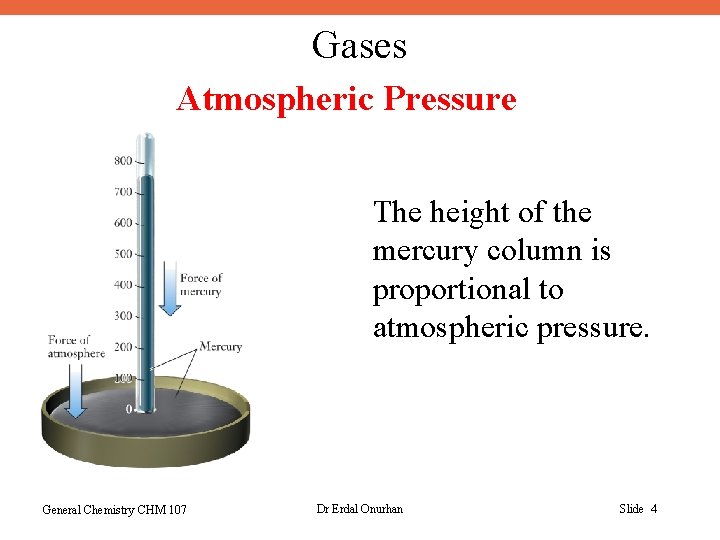
Gases Atmospheric Pressure The height of the mercury column is proportional to atmospheric pressure. General Chemistry CHM 107 Dr Erdal Onurhan Slide 4

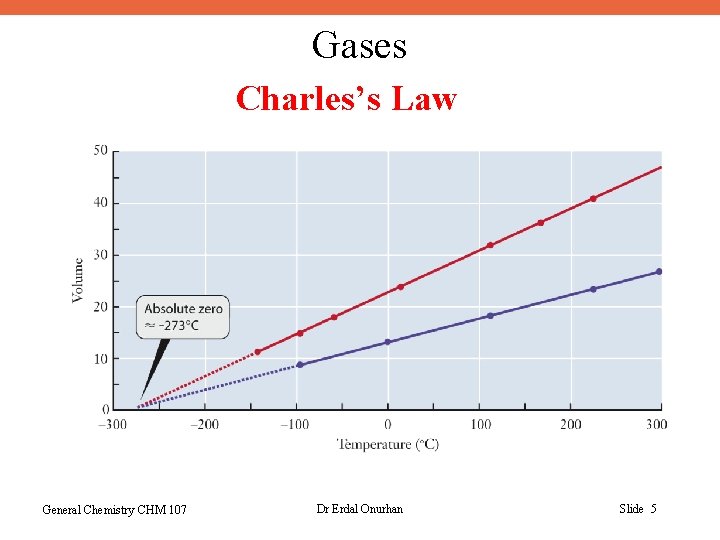
Gases Charles’s Law General Chemistry CHM 107 Dr Erdal Onurhan Slide 5

Gases Postulates of Kinetic Molecular Model a) Gases are made up of large collections of particles, which are in constant, random motion b) Gas particles are infinitely small and occupy negligible volume c) Gas particles move in straight lines except when they collide with other particles or with the container walls. These collisions are elastic, so kinetic energy of particles is conserved d) Particles interact with each other only when collisions occur e) The average kinetic energy of a gas is proportional to the absolute temperature of the gas but does not depend upon the identity of the gas General Chemistry CHM 107 Dr Erdal Onurhan Slide 6