FEMALE FERTILITY PRESERVATON OPTIONS Female Fertility Option Standard







- Slides: 13

FEMALE FERTILITY PRESERVATON OPTIONS

Female Fertility Option • Standard of Care Options • Oocyte / Embryo Cryopreservation • Ovarian Transposition • Experimental Options • Gn. RH Analogues • Ovarian Cryopreservation

STANDARD OF CARE OPTIONS

Oocyte & Embryo Cryopreservation Consideration in Pediatric & Adolescent Patients • Standard of Care options in post-pubertal female patients • Oocyte cryopreservation non-experimental (ASRM 2012) • Controlled ovarian stimulation multiple mature oocytes • Success rates published by clinic • Society for Assisted Reproductive Technologies • Average: 33 -52% live birth rates • Limited adolescent and oncology specific data • Barriers: Time/delay in treatment, cost/insurance coverage, concern for procedural technique or side effects • Consideration: Adolescent friendly alterations Mature oocyte cryopreservation: A Guideline. Fertil Steril 2013; 99: 37– 43

Ovarian Transposition • Pelvic or abdominal radiation with significant risk of amenorrhea and subsequent infertility. • greater than 15 Gy in prepubertal girls • greater than 10 Gy in postpubertal girls • Surgically move ovaries out of the radiation field • Success rates • • 50 -90% reduction in radiation to the ovary 30 -50% maintenance of ovarian function Unknown fertility preservation success Not well study in pediatric population Estes S End Met 2015, https: //www. cincinnatichildrens. org/service/f/fertility-preservation/females

EXPERIMENTAL OPTIONS

Gn. RH Analogues • Mimic pre-pubertal “quiescent” ovary • Problems with early study design • More recent studies improve design • 3 show decreased amenorrhea • 3 show no protection of ovarian reserve (2 stopped earlier) • 2013 ASCO Guidelines: Insufficient evidence • POEMS Trial (2015) • Breast Cancer + Goserelin • Reduction in ovarian failure (p = 0. 04) • Increased odds ratio for pregnancy (p = 0. 03) Levine J Children 2014, Loren AW et al J Clin Oncol 2013, Moore HCF et al J Clin Onc 2014.

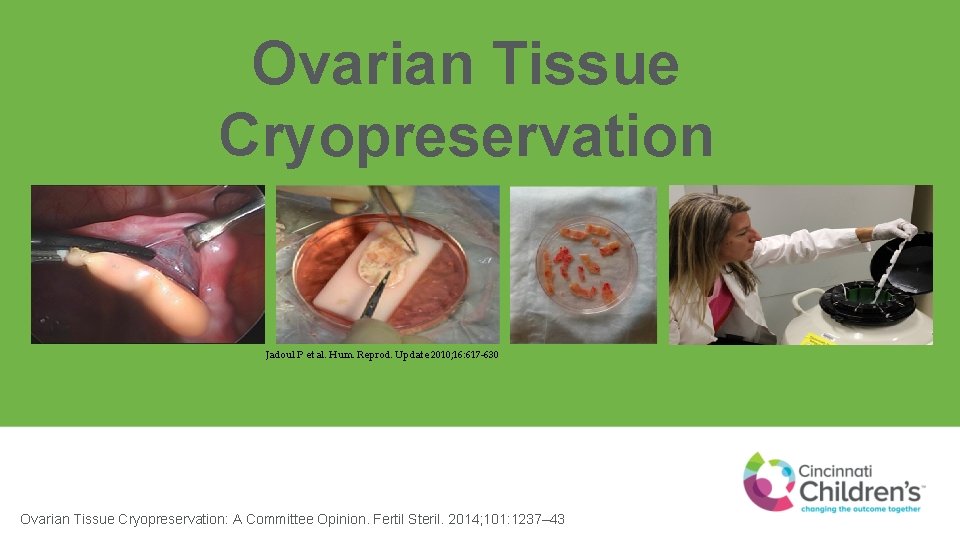
Ovarian Tissue Cryopreservation Jadoul P et al. Hum. Reprod. Update 2010; 16: 617 -630 Ovarian Tissue Cryopreservation: A Committee Opinion. Fertil Steril. 2014; 101: 1237– 43

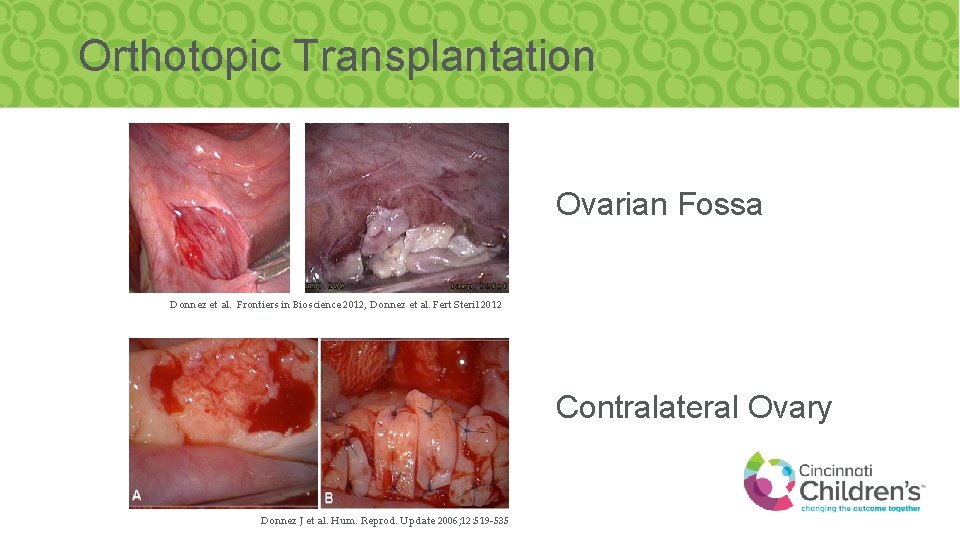
Orthotopic Transplantation Ovarian Fossa Donnez et al. Frontiers in Bioscience 2012, Donnez et al. Fert Steril 2012 Contralateral Ovary Donnez J et al. Hum. Reprod. Update 2006; 12: 519 -535

Donnes et al. , 2013, 2016; Dittrich et al. , 2015; Jensen et al. , 2015; Meirow et al. , Van der en et al. , 2016

CCHMC OTC Protocols • Females 1 month to 41 years of age • Undergo surgery, chemotherapy, drug treatment, and/or radiation for the • treatment or prevention of a medical condition or Purposely left broad malignancy expected to result in permanent and complete loss of • No restrictions based on risk subsequent ovarian function • Or, haveassessment a medical condition or malignancy that requires removal of all or part of one both ovaries. • Allows fororcase by case evaluation • Is not a candidate for or chooses not to utilize embryo or oocyte banking. • Serum FSH levels ≤ 20 m. IU/ml.

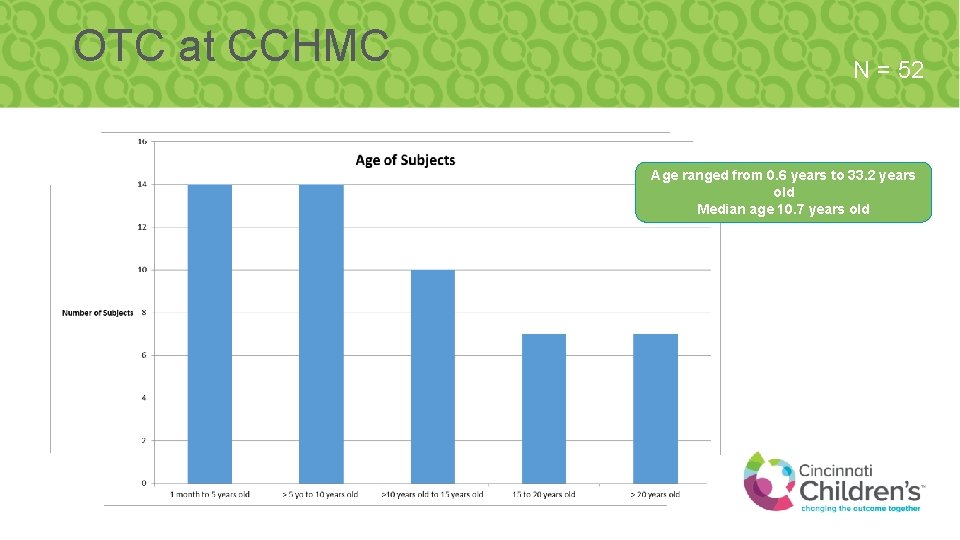
OTC at CCHMC N = 52 Age ranged from 0. 6 years to 33. 2 years old Median age 10. 7 years old

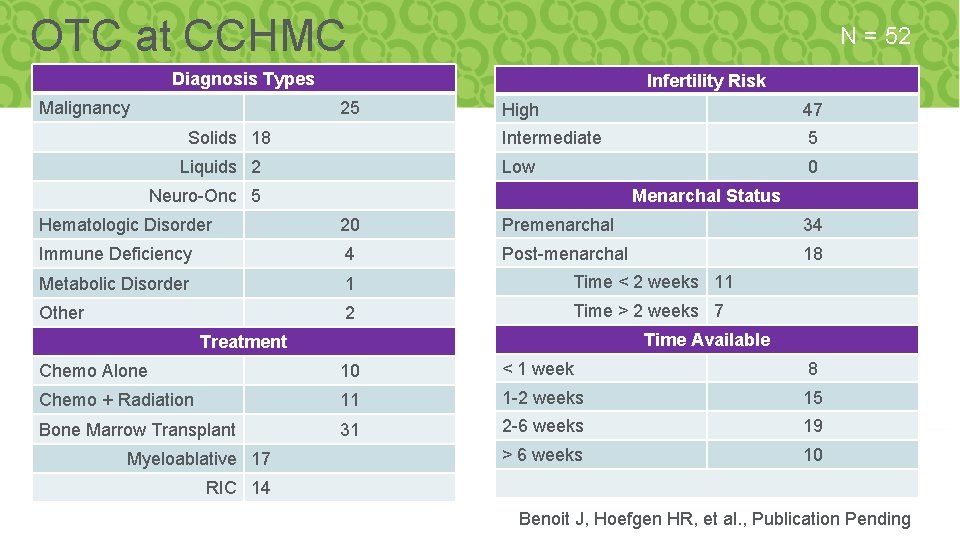
OTC at CCHMC N = 52 Diagnosis Types Malignancy Infertility Risk 25 Solids 18 Liquids 2 High 47 Intermediate 5 Low 0 Neuro-Onc 5 Menarchal Status Hematologic Disorder 20 Premenarchal 34 Immune Deficiency 4 Post-menarchal 18 Metabolic Disorder 1 Time < 2 weeks 11 Other 2 Time > 2 weeks 7 Time Available Treatment Chemo Alone 10 < 1 week 8 Chemo + Radiation 11 1 -2 weeks 15 Bone Marrow Transplant 31 2 -6 weeks 19 > 6 weeks 10 Myeloablative 17 RIC 14 Benoit J, Hoefgen HR, et al. , Publication Pending (n= 52)
 Soal pilihan ganda (multiple choice)
Soal pilihan ganda (multiple choice) Option a option b
Option a option b Solaray fertility blend
Solaray fertility blend Standard deviation options
Standard deviation options Language
Language Objective of standard costing
Objective of standard costing Bahagian pembangunan kurikulum dskp 2020
Bahagian pembangunan kurikulum dskp 2020 Standard error for mean
Standard error for mean Contoh rumus tfr
Contoh rumus tfr General fertility rate
General fertility rate Fertility capability classification
Fertility capability classification Birth rate decline
Birth rate decline General fertility rate
General fertility rate General fertility rate
General fertility rate