Faculty of Pharmacy Department of Pharmaceutical Chemistry Practical


- Slides: 8

Faculty of Pharmacy Department of Pharmaceutical Chemistry Practical Analytical Chemistry (1) Practical (3)

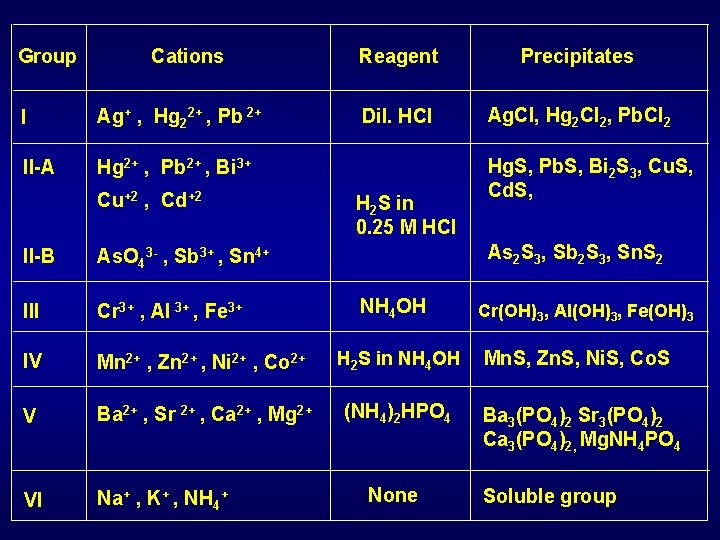
Group Cations I Ag+ , Hg 22+ , Pb 2+ II-A Hg 2+ , Pb 2+ , Bi 3+ Cu+2 , Cd+2 Reagent Precipitates Dil. HCl Ag. Cl, Hg 2 Cl 2, Pb. Cl 2 H 2 S in 0. 25 M HCl Hg. S, Pb. S, Bi 2 S 3, Cu. S, Cd. S, As 2 S 3, Sb 2 S 3, Sn. S 2 II-B As. O 43 - , Sb 3+ , Sn 4+ III Cr 3+ , Al 3+ , Fe 3+ IV Mn 2+ , Zn 2+ , Ni 2+ , Co 2+ H 2 S in NH 4 OH Mn. S, Zn. S, Ni. S, Co. S V Ba 2+ , Sr 2+ , Ca 2+ , Mg 2+ (NH 4)2 HPO 4 Ba 3(PO 4)2 Sr 3(PO 4)2 Ca 3(PO 4)2, Mg. NH 4 PO 4 VI Na+ , K+ , NH 4+ NH 4 OH None Cr(OH)3, Al(OH)3, Fe(OH)3 Soluble group

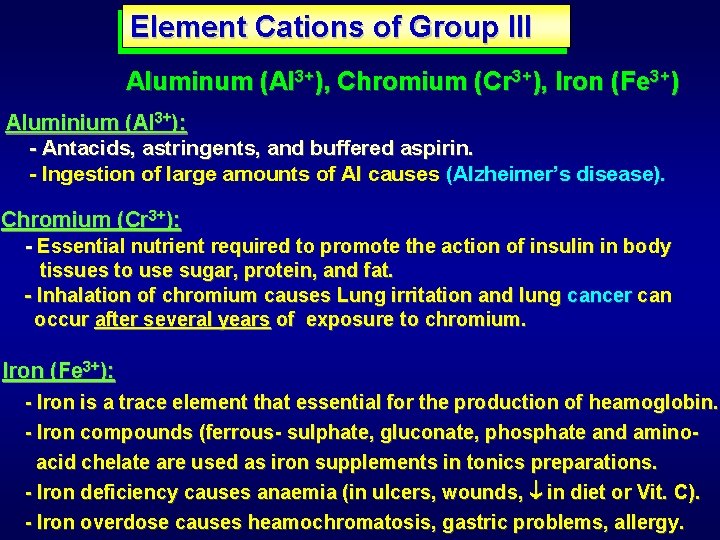
Element Cations of Group III Aluminum (Al 3+), Chromium (Cr 3+), Iron (Fe 3+) Aluminium (Al 3+): - Antacids, astringents, and buffered aspirin. - Ingestion of large amounts of Al causes (Alzheimer’s disease). Chromium (Cr 3+): - Essential nutrient required to promote the action of insulin in body tissues to use sugar, protein, and fat. - Inhalation of chromium causes Lung irritation and lung cancer can occur after several years of exposure to chromium. Iron (Fe 3+): - Iron is a trace element that essential for the production of heamoglobin. - Iron compounds (ferrous- sulphate, gluconate, phosphate and aminoacid chelate are used as iron supplements in tonics preparations. - Iron deficiency causes anaemia (in ulcers, wounds, in diet or Vit. C). - Iron overdose causes heamochromatosis, gastric problems, allergy.

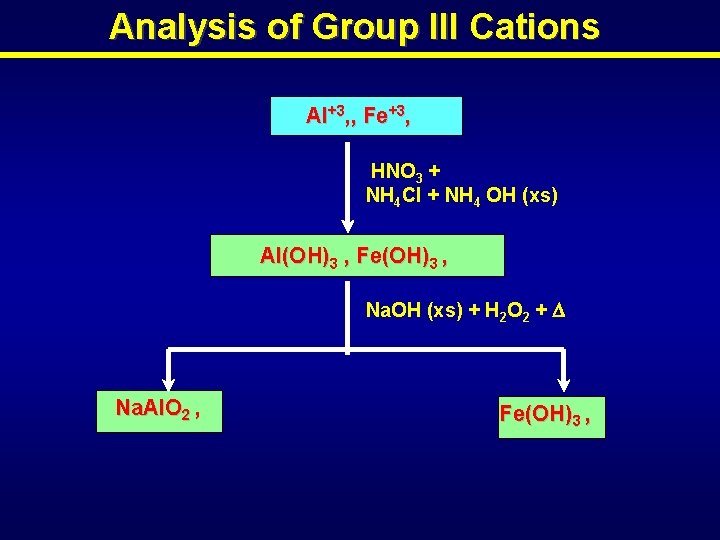
Analysis of Group III Cations Al+3, , Fe+3, HNO 3 + NH 4 Cl + NH 4 OH (xs) Al(OH)3 , Fe(OH)3 , Na. OH (xs) + H 2 O 2 + Na. Al. O 2 , Fe(OH)3 ,

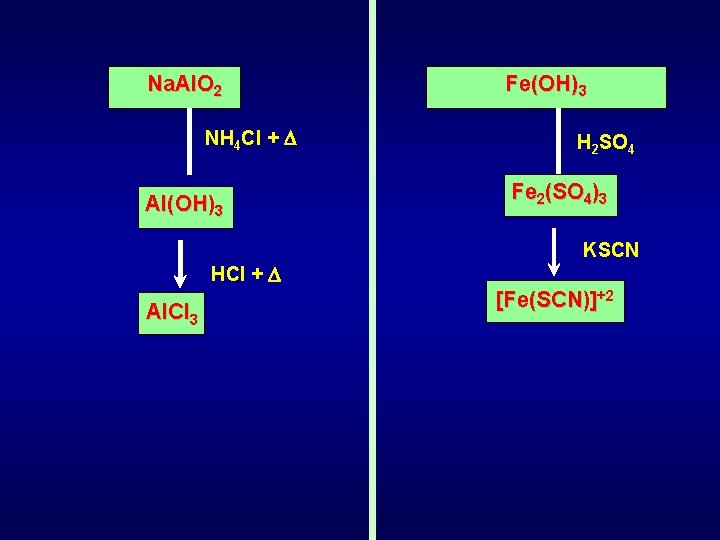
Na. Al. O 2 NH 4 Cl + Al(OH)3 HCl + Al. Cl 3 Fe(OH)3 H 2 SO 4 Fe 2(SO 4)3 KSCN [Fe(SCN)]+2

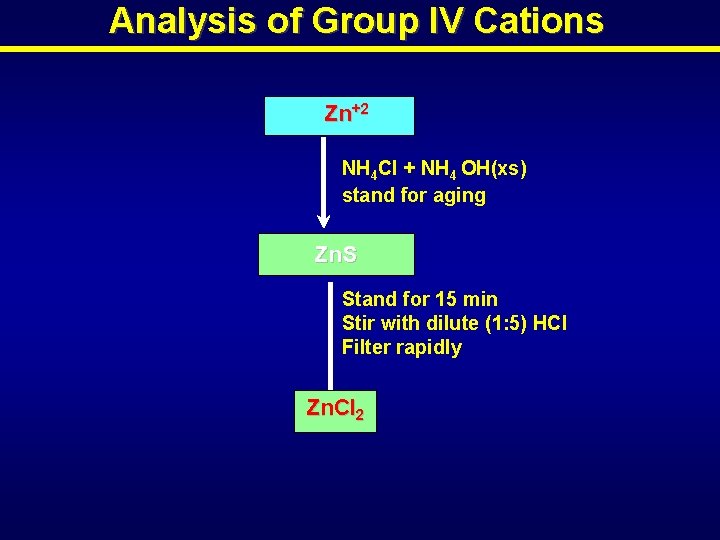
Analysis of Group IV Cations Zn+2 NH 4 Cl + NH 4 OH(xs) stand for aging Zn. S Stand for 15 min Stir with dilute (1: 5) HCl Filter rapidly Zn. Cl 2

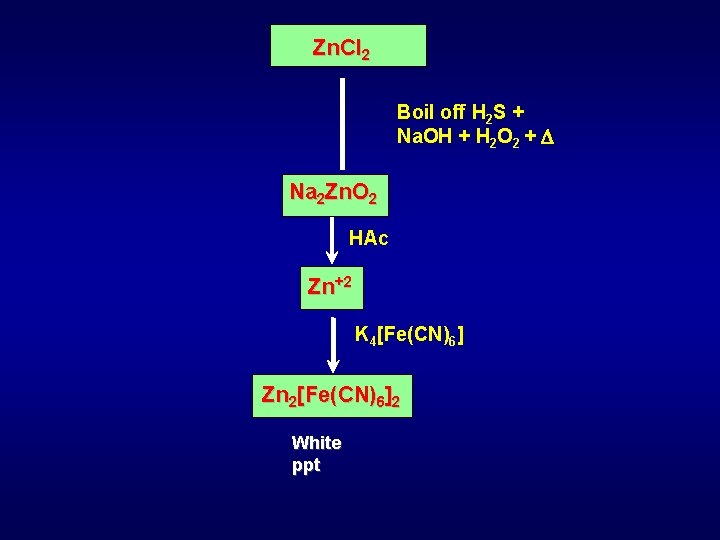
Zn. Cl 2 Boil off H 2 S + Na. OH + H 2 O 2 + Na 2 Zn. O 2 HAc Zn+2 K 4[Fe(CN)6] Zn 2[Fe(CN)6]2 White ppt

Points for discussion 1 -Write a flow chart for your unknown analysis in your lab notebook. You should include formulas of all reagents added, colors of all solutions and ppts, and conclusions made from these observations. For example, your original solution is colorless, you add HCl forming a white ppt and a colorless solution. At this point you do not know what the formula of the white ppt is, so do not write one. 2. What cation is present in your unknown















