Energy Enzymes Energy All living things need a

















- Slides: 17

Energy & Enzymes

Energy All living things need a source of, and use, energy Energy is used in series of reactions called metabolism The amount of energy in the universe remains the same over time.

Law of Conservation of Energy CANNOT be created or destroyed… …it can only CHANGE form.

Chemical Reactions A process that changes one set of chemicals into another set of chemicals. Can be Slow Ex: Iron + Oxygen rust Fast Ex: Hydrogen + Oxygen explosion

Chemical Reactions Reactants on the left Products on the right CO 2 + H 2 O H 2 CO 3

Vocabulary Activation Energy: Amount of energy needed to start a reaction Catalyst: anything that lowers the amount of activation energy needed Unchanged or consumed during a reaction

Enzymes PROTEIN catalyst found in living things (biological catalysts) HINT: Enzymes generally end in -ase (Ligase, primase, lactase) Enzymes work as lock and key Image from: http: //www. cas. muohio. edu/~wilsonkg/old/gene 2005/syllabus_F 03_23. jpg

Enzymes are made of proteins. Ø They help speed up reactions and are UNCHANGED by the reaction. Ø Shape changes, enzyme can no longer function Ø Image from: http: //www. cas. muohio. edu/~wilsonkg/old/gene 2005/syllabus_F 03_23. jpg

Enzyme Specificity Lock and key: each enzyme is specific lock that only fits 1 key (substrate) Substrate= reactant on which enzyme acts Area on the enzyme to which substrate “fits” is the active site

How enzymes work

Protein Denaturation Denatured: To change a protein’s shape Example: scrambled eggs Recall that enzyme activity depends on shape…

What can denature a protein? Change in temperature (too hot, too cold) Change in p. H


Chemical Reactions + Energy Every chemical reaction involves a transfer of energy Can be: Exergonic: release of energy Endergonic: absorption of energy

Exergonic Reaction Products have less energy than the reactants Released as heat, light, or electricity Examples: Glow sticks, Hot hands (Exothermic)

Endergonic Reaction Products have more energy than the reactants Energy is absorbed! Example: photosynthesis

Enzyme inhibition Inhibitors: substance that prevents enzymes from working Competitive inhibition: Competes with substrate Noncompetitive inhibitors: Binding changes active site
 What is the smallest living unit
What is the smallest living unit What are the seven life processes of living things
What are the seven life processes of living things Why are enzymes important to living things
Why are enzymes important to living things Pyramid hesd
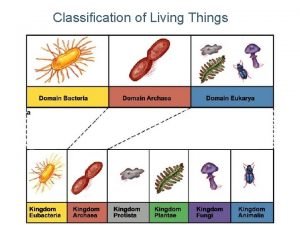
Pyramid hesd Why do we classify things
Why do we classify things Cells group together to form
Cells group together to form Life cycle of all living things
Life cycle of all living things What are the 5 basic needs of all living things
What are the 5 basic needs of all living things Different types of living organisms
Different types of living organisms All living things 1955
All living things 1955 Enzyme catalyzed reaction
Enzyme catalyzed reaction How many links are there in a food chain
How many links are there in a food chain Third level consumer
Third level consumer Gets its energy from eating living things
Gets its energy from eating living things How living things obtain energy worksheet answers
How living things obtain energy worksheet answers How living things obtain energy worksheet answers
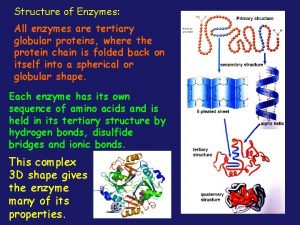
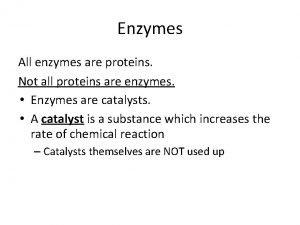
How living things obtain energy worksheet answers All enzymes are globular proteins
All enzymes are globular proteins Not all enzymes are proteins
Not all enzymes are proteins