Effect of Temperature on Catalase Activity Adham Ahmed







- Slides: 12

Effect of Temperature on Catalase Activity Adham Ahmed, Amy Jacob, Arshia Iqbal, Jaime Peter

Research Question What is the effect of temperature (27 ℃, 37 ℃, 47 ℃) on potato (Solanum Tuberosum) catalase activity as measured by a Vernier gas pressure sensor?

Introduction ● The fundamental role of proteins is to act as enzymes. ● Catalystsare enzymes that speed up a reaction by lowering the activation energy. ● Catalysts are needed in our body to decompose hydrogen peroxide, a toxic byproduct of metabolism. If hydrogen peroxide is left in the body, it can damage cells. ● We used a Vernier gas pressure sensor to observe the difference in the initial and final pressures of a potato hydroxide mash at different temperatures.

Hypothesis ❖ If the temperature of the solution containing hydrogen peroxide and potato catalase increases, then the potato catalase activity would increase because an increase in temperature causes an increase in entropy. “Entropy is the measure of the disorder of a system. ” This results in substrates and enzymes to move faster and collide more often, therefore speeding up the rate of reaction.

Procedure ● Preparation of potato catalase ○ ○ Blended potato cubes into a mash. Strained the mash to obtain the liquid with the potato catalase ● Conducting the experiment ○ ○ ○ Mixed 5 m. L of 3% hydrogen peroxide and 1 m. L of potato catalase Placed the test tube in a beaker which was on top of a hot plate The beaker was then heated on a hot plate, heating the potato catalase and hydrogen peroxide solution to 27℃. Measured the initial pressure, waited for a minute and then recorded the final pressure. ■ Repeated 2 more times for 27℃ 3 trials were conducted and recorded for 37℃ and 47℃ respectively.

Pictures

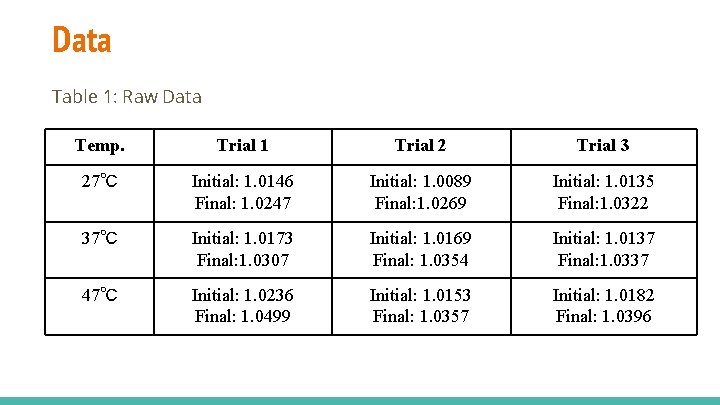
Data Table 1: Raw Data Temp. Trial 1 Trial 2 Trial 3 27℃ Initial: 1. 0146 Final: 1. 0247 Initial: 1. 0089 Final: 1. 0269 Initial: 1. 0135 Final: 1. 0322 37℃ Initial: 1. 0173 Final: 1. 0307 Initial: 1. 0169 Final: 1. 0354 Initial: 1. 0137 Final: 1. 0337 47℃ Initial: 1. 0236 Final: 1. 0499 Initial: 1. 0153 Final: 1. 0357 Initial: 1. 0182 Final: 1. 0396

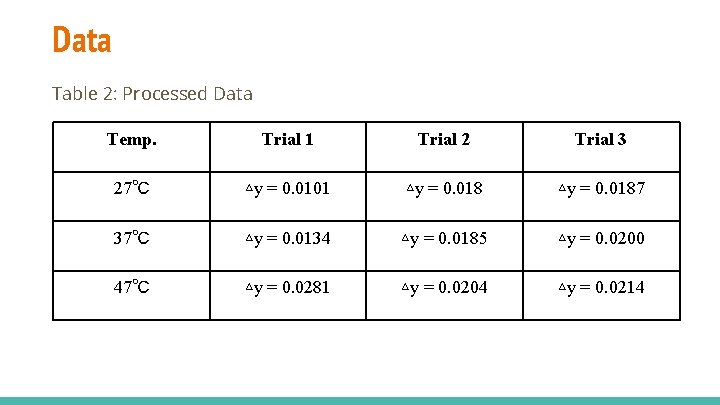
Data Table 2: Processed Data Temp. Trial 1 Trial 2 Trial 3 27℃ ▵y = 0. 0101 ▵y = 0. 0187 37℃ ▵y = 0. 0134 ▵y = 0. 0185 ▵y = 0. 0200 47℃ ▵y = 0. 0281 ▵y = 0. 0204 ▵y = 0. 0214

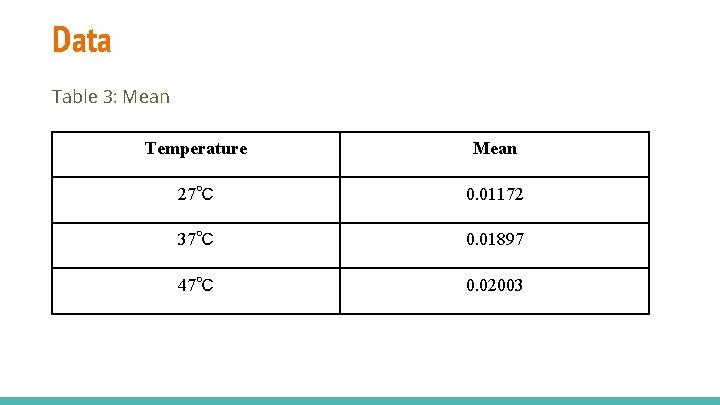
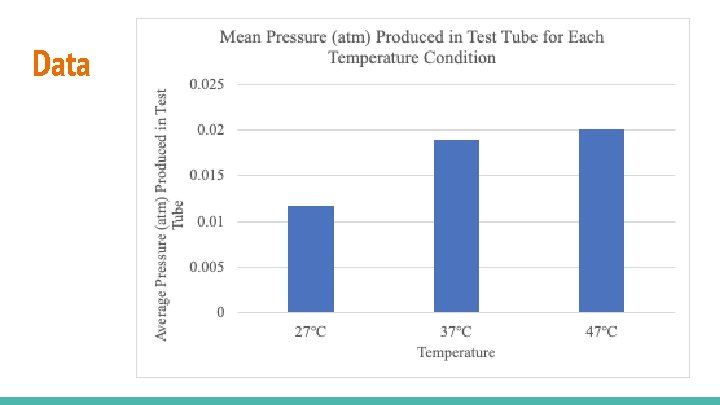
Data Table 3: Mean Temperature Mean 27℃ 0. 01172 37℃ 0. 01897 47℃ 0. 02003

Data

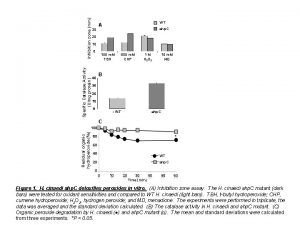
Conclusion/Discussion - Hypothesis is supported; there is direct relationship between temperature and enzymatic activity. As temperature rose, there was increase in mean gas pressures. (table 4, figure 3). This shows that the catalase was more active at higher temperatures and the rate of reaction was greater, Possible Sources of Error 1. Prolonged use of blender 2. Fluctuation of room temperature during experiments.

Citations Clark, J. (2012). The Effect of Temperature on Reaction Rates. Retrieved from http: //chemguide. co. uk/physical/basicrates/temperature. html Coniel, O. (n. d. ). IB Guides. Retrieved from http: //ibguides. com/biology/notes/enzymes Toxic Substances Portal - Hydrogen Peroxide. (2014, October 21). Retrieved from https: //www. atsdr. cdc. gov/MMG. asp? id=304&tid=55