Dyeing of Wool Silk Nylon and Acrylic Faiza













- Slides: 18

Dyeing of Wool, Silk, Nylon and Acrylic Faiza Anwar

Acid Dyes • So called because these are usually applied under acidic conditions. • Dyes are normally very large aromatic molecules consisting of many linked rings. • Acid dyes usually have a carboxyl or amino group on the molecule. • General formula DSO 3 -Na+ i-e sodium salt of sulphonic acid

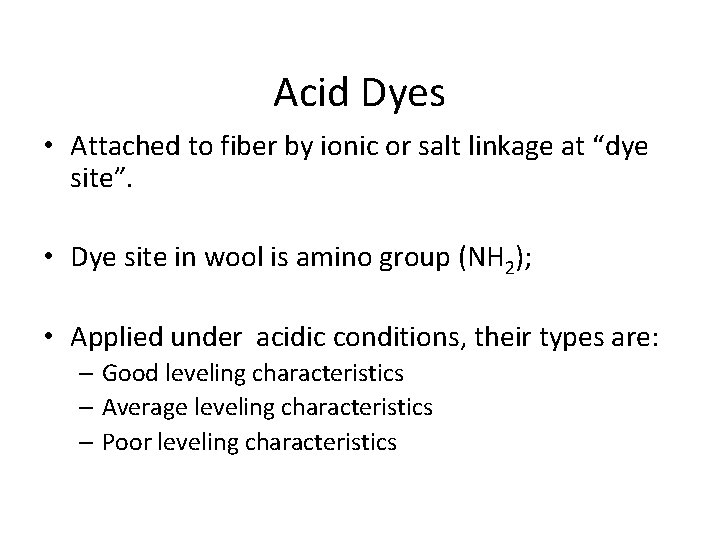
Acid Dyes • Attached to fiber by ionic or salt linkage at “dye site”. • Dye site in wool is amino group (NH 2); • Applied under acidic conditions, their types are: – Good leveling characteristics – Average leveling characteristics – Poor leveling characteristics

Types of Acid Dyes • Good leveling – They have poor substantivity that’s why they have good levelling property. – applied in 3. 5 -4. 5 ph. • Average leveling – They have average substantivity that’s why they have average levelling property. – applied in 5 -6 p. H, • Poor leveling characteristics • They have very good substantivity that’s why they have poor levelling property. – applied in 6 -7 p. H.


Acid Dyes • Dark shades achievable on wool b/c of its amorphous nature and plenty of amino groups. • Same is the mechanism for silk and synthetics like polyamide • Synthetic fibers may form an ionic or salt linkage. • They are highly substantive dyes so uneven dyeing may result • Evenness achieved by use of retarders such as Na 2 SO 4.

Dyeing with Acid Dyes • Applied under acidic conditions maintained by the addition of – sulphuric acid in dye solution for good leveling dyes – Acetic Acid in dye solution for average and poor leveling dyes • Good substantivity (so chances of unlevelness) • Sodium Sulphate is used as a Retarder for level dyeing.

Addition of Acid • Addition of acid acts as an exhausting agent, because strongly acidic conditions makes more cationic sites available and thus available dye anions got combined with these.

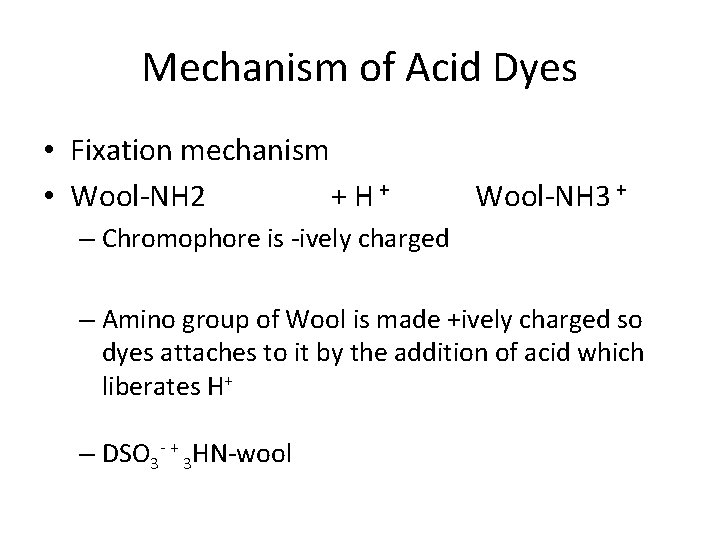
Mechanism of Acid Dyes • Fixation mechanism • Wool-NH 2 + H+ Wool-NH 3 + – Chromophore is -ively charged – Amino group of Wool is made +ively charged so dyes attaches to it by the addition of acid which liberates H+ – DSO 3 - + 3 HN-wool

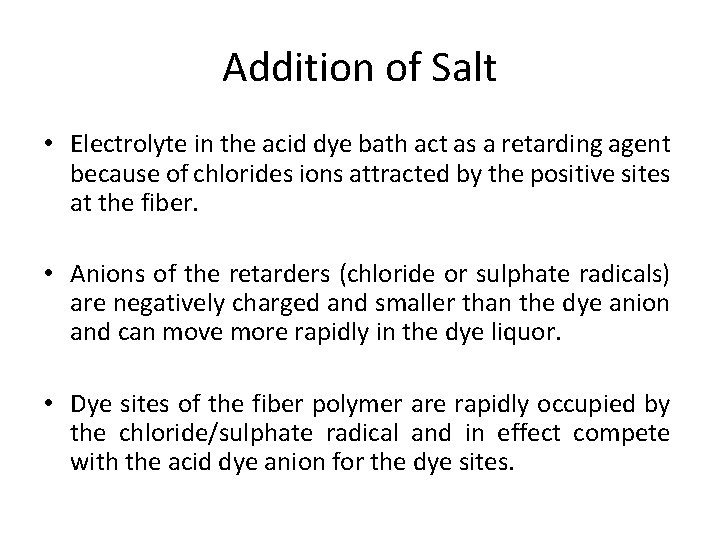
Addition of Salt • Electrolyte in the acid dye bath act as a retarding agent because of chlorides ions attracted by the positive sites at the fiber. • Anions of the retarders (chloride or sulphate radicals) are negatively charged and smaller than the dye anion and can move more rapidly in the dye liquor. • Dye sites of the fiber polymer are rapidly occupied by the chloride/sulphate radical and in effect compete with the acid dye anion for the dye sites.

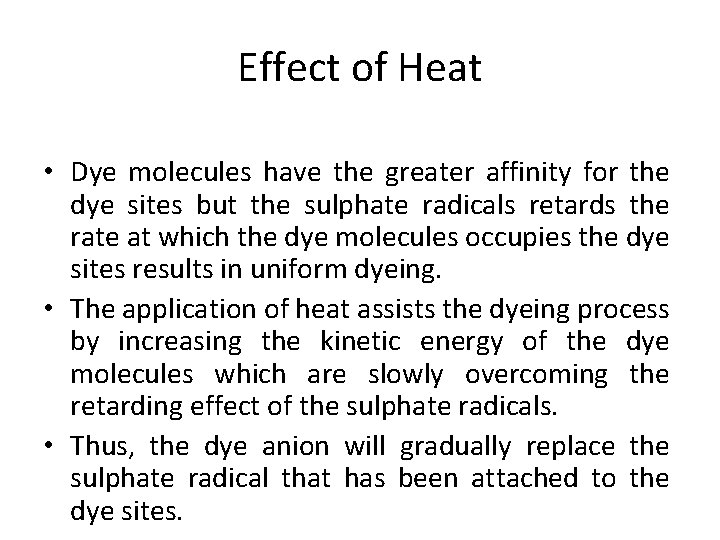
Effect of Heat • Dye molecules have the greater affinity for the dye sites but the sulphate radicals retards the rate at which the dye molecules occupies the dye sites results in uniform dyeing. • The application of heat assists the dyeing process by increasing the kinetic energy of the dye molecules which are slowly overcoming the retarding effect of the sulphate radicals. • Thus, the dye anion will gradually replace the sulphate radical that has been attached to the dye sites.

Properties of Acid Dyes • Light fastness is good being rating of about 5. – Since the chromophore of acid dyes are stable and can bear the UV exposure present in sunlight. – • Wash fastness is poor to good depending upon substantivity of the type of dye used. – W. F is 2 -3 with good leveling characterstics – 3 -4 for those whose average leveling – 4 -5 for poor leveling characterstics.

Reasons for Low wash fastness – Firstly, This is because the ionic and hydrogen bonds are hydrolyzed in water. – Secondly these dyes are acidic in nature so can not bear alkaline treatments during washing and laundering.

Basic Dyes • Can be used for wool, silk and acrylic and modacrylic • But poor properties for fibers other than acrylic • Also called cationic dyes b/c they acquire +ive charge in solution • Are MOST BRILLIANT dyes

Dyeing with Basic Dyes • Applied under slight acidic solution normally 6 -7. • Good substantivity (so chances of unlevelness) • Retarder used for level dyeing

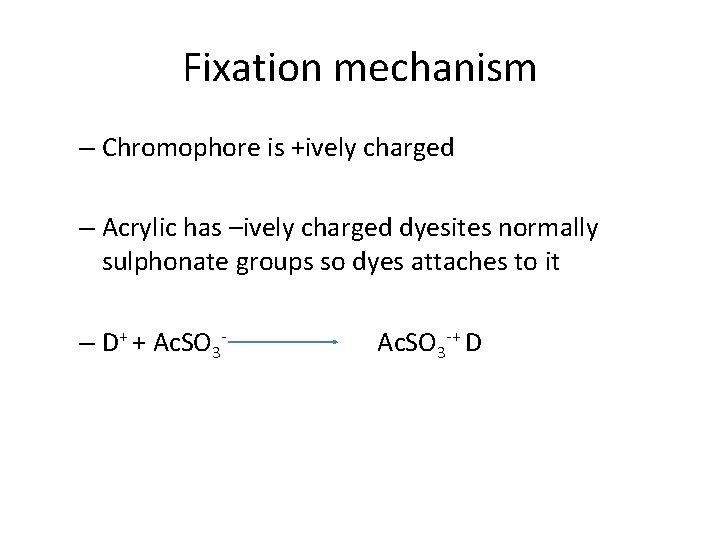
Fixation mechanism – Chromophore is +ively charged – Acrylic has –ively charged dyesites normally sulphonate groups so dyes attaches to it – D+ + Ac. SO 3 -+ D

Properties of Basic Dyes • Light fastness is excellent 6 -7 • Wash fastness is very good 4 -5 – Due to high substantivity and hydrophobicity of acrylic • Brightness – Excellent brilliance and color intensity

References • Textile Science, by: E. P. G Gohl
 Acrylic vs polyester
Acrylic vs polyester Woollen textile industry
Woollen textile industry Silk is a natural protein fiber some forms of which can be
Silk is a natural protein fiber some forms of which can be Lahalibo 1949
Lahalibo 1949 Pemra cable tv licence fee
Pemra cable tv licence fee Faiza asghar
Faiza asghar Hyperparathyroidism causes
Hyperparathyroidism causes Faiza amjad
Faiza amjad Faiza anwar
Faiza anwar Features of victorian era
Features of victorian era Faiza bhatti
Faiza bhatti Acetate fabric advantages and disadvantages
Acetate fabric advantages and disadvantages Matrix acrylic aurium
Matrix acrylic aurium Servsafe acrylic nails
Servsafe acrylic nails One piece casting denture
One piece casting denture Stages of self cure acrylic resin
Stages of self cure acrylic resin Internal finishing line rpd
Internal finishing line rpd Emulsion acrylic adhesive
Emulsion acrylic adhesive Posh
Posh