Dr C Mercy Assistant professor PG Department of























- Slides: 25

Dr. C. Mercy, Assistant professor, PG Department of zoology, Sarah Tucker College, Tirunelveli.

BETAOXIDATION

Introduction v In biochemistry , beta-oxidation is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-Co. A, which enters the citric acid cycle, and NADH and FADH 2, which are co-enzymes used in the electron transport chain. v v It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

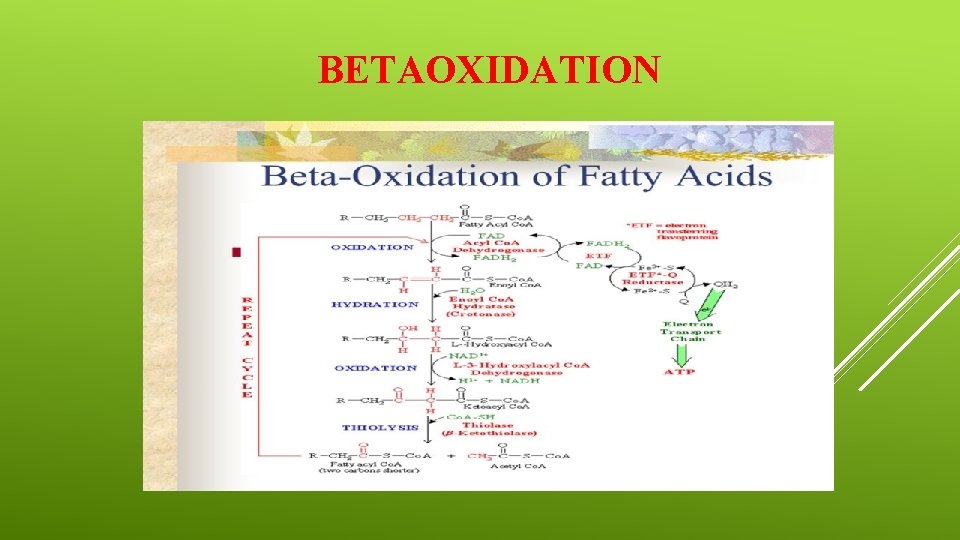
Beta-oxidation v It was discovered by F. Knoop in the beginning of twentieth century. v Initially this process was discovered and explained in animal systems. v v Later on, the enzymes involved in the process were also discovered in plants. (i)The initial step in B-oxidation is the activation of a fatty acid by its information into the corresponding Co. Athioester with the help of coenzymes A. v The energy required for this process is derived from ATP. v This reaction has been found to catalyzed by the appropriate acyl Co. A synthetase (an enzyme).

v Many such enzymes are known which have been named according to the length of the carbon chain of the fatty acid that reacts most rapidly. v Generally, two types of such enzymes are known, one specific for medium length carbon chains (4 Cto 12 C) and the other for longer chains. v For example, the enzyme from Bacillus megatherium reacts with fatty acids of 6 to 20 carbon atoms. v Another mechanism for the synthesis of an acyl-Co. A derivatives involves the transfer reaction catalyzed by thiophorases. v It is a transacetylation reaction wherein Co. A gets transferred from propionyl-Co. A or succinyl-Co. A to the appropriate fatty acid:

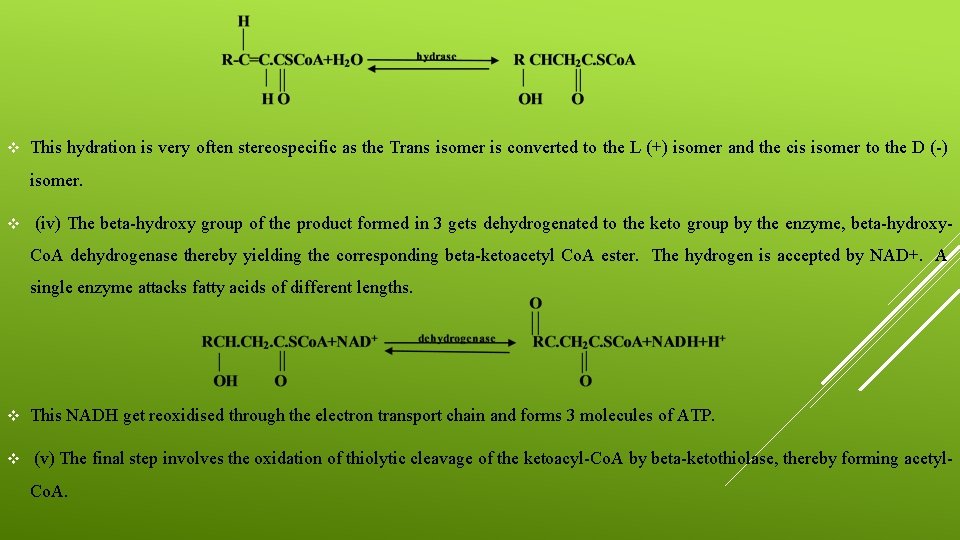
v (ii)The product formed in the initial step (i) undergoes dehydrogenation, i. e. two hydrogen atoms are removed from alpha and beta-unsaturated fatty acyl-Co. A derivatives. v They require the coenzyme FAD and enzyme, acyl-Co. A dehydrogenase. v Three types of dehydrogenases have been identified from liver tissues. v They have been found to differ according to their specificity to the substrate. v The first has been found to be acting on long chain fatty acids (C 14, C 16, C 18), Whereas the other two have been found to be acting on medium or short chains. v (iii)The product formed in the step (ii) involves addition of water in the presence of the enzyme enoyl-Co. A hydrase and yields a beta-hydroxyacyl-Co. A derivative.

v This hydration is very often stereospecific as the Trans isomer is converted to the L (+) isomer and the cis isomer to the D (-) isomer. v (iv) The beta-hydroxy group of the product formed in 3 gets dehydrogenated to the keto group by the enzyme, beta-hydroxy. Co. A dehydrogenase thereby yielding the corresponding beta-ketoacetyl Co. A ester. The hydrogen is accepted by NAD+. A single enzyme attacks fatty acids of different lengths. v This NADH get reoxidised through the electron transport chain and forms 3 molecules of ATP. v (v) The final step involves the oxidation of thiolytic cleavage of the ketoacyl-Co. A by beta-ketothiolase, thereby forming acetyl. Co. A.

v In this reaction the product is a fatty acid with two carbon atoms short. v In may inter the entire process of oxidation at step (ii) and further dissociation may take place. v Hence, it is a process of cleaving fatty acids into fragments of 2 C compounds in steps In summary the shortening of fatty acyl-Co. A two carbon atoms can be represented by the following equation:


v The beta-oxidation of palmitic acid is illustrated. v Beta-oxidation of fats is a good source of energy production. v In each cycle of beta-oxidation, there occurs the formation of 5 ATP molecules (3 from the oxidation of NADH and 2 from FADH 2). v One ATP gets used up in the reaction. In this way 35 -1=34 ATP molecules are formed in 7 turns of palmitic acid oxidation. v Besides this, 8 acyl Co. A produced from palmitic acid will yield 12 x 8=96 ATP molecules. v This will give a total of 130(96+34) ATP per palmitic acid molecules.

v v v The end produced of beta-oxidation of fatty acid is acetyl-Co. A. Fatty acids having odd number of carbon atoms from acetyl-Co. A and propionyl-Co. A. The acetyl-Co. A has been either oxidized for the production of ATP or converted into carbohydrates. The propionyl Co. A gets converted to malonic semialdehyde in both plants and animals. Malonic semialdehyde could be converted to alanine by transamination with glutamic acid in animals. In plants, however, this compound gets oxidized to CO 2 and H 2 O.

BIOSYNTHESIS OF SATURATED FATTY ACIDS Introduction v It was belied for long that fatty biosynthesis was the reversal of the pathway of fatty acid degradation. v However, several major points of difference between the degradation and biosynthesis of fatty acids are known. Biosynthesis v (i) Co. A- derivatives do not act as the substrates of the enzymes in fatty acid synthesis but instead acyl moieties are connected to an acyl carrier protein (ACP). v ACP has a molecular weight of 10, 000. v Its prosthetic group is 4 -phosphopantheine and it thus resembles coenzymes.


v (ii) In biosynthesis, the basic “adding unit” is malonyl-Co. A but not acetyl-Co. A. v (iii) Biosynthesis uses NADPH while NAD is involved in oxidation. In addition ATP and bicarbonate are also employed. v (iv) In biosynthesis, the hydroxy acids involved are D (-)-beta isomers while the L (+)-beta isomers occur in oxidation. v Even numbered saturated fatty acid are synthesized by a series of reaction in which 2 carbon units derived from malonyl-ACP molecule. v The initial reactions in fatty acid synthesis involves the formation of acyl-ACP and malonyl-ACP.

v The formation of malonyl-Co. A has been catalyzed by the biotin containing enzyme called acetyl-Co-A carboxylase. v In some microorganisms, malonyl-Co. A can be formed by reactions other than carboxylation of acetyl Co. A. v These reactions are as follows: v (i) Activation of malonate by the specific malonyl-Co. A sythetase in the presence of ATP and Co. A. Malonate + Co. ASH + ATP v malonyl –Co. A+ADP+Pi (ii) Co. A transferase reaction between malonate and succinyl-Co. A or acetoacetyl-Co. A. R – CO – SCo. A + malonate malonyl – Co. A + RCOOH and v (iii) Oxidation of malonyl - semi aldehyde to malonyl Co. A. v An attempt was made to study fatty acid sythesising systems from a number of resources such as E. coli, yeast and pigeon liver.

v In E. coli, there are seven different enzymes which are involved in each turn of a cycle, so as to extend the fatty acyl chain. v There of these are acetyl transacetylase, acetyl Co. A carboxylase and malonyl transacetylase which are involved in the formation of acetyl – ACP and malonyl – ACP. v Four additional enzymes are needed to elongate acyl – SACP by two carbon atoms to form a 4 -carbon acyl fragment, the butyrylacyl-SACP. v These steps are as follows: v (i) Firstly, acetyl-ACP reacts with malonyl ACP to form acetoacetyl –SACP in the presence of 3 - ketoacyl SACP sythetase.

v (ii) Secondly, acetoacetyl-SACP is reduced to D (-)-beta-hydroxybutyryl-SACP by the enzyme 3 -ketoacyl SACP reductase. v (iii) Dehydration of D (-)-beta-hydroxybutyryl- SCAP with the enzyme beta-hydroxyacyl-ACP dehydrogenase yields crotonyl-SACP. v v (iv) Reduction of crotonyl-SCAP with the enzyme, enoyl-ACP reductase yields butyryl-SACP. The butyryl-ACP is then recycled and then condensed with another malonyl ACP to from a 6 -carbon fragment. v The essential steps involved in fatty acid biosynthesis via the malonyl are given.


Introduction v PALMITIC ACID Palmitic acid, or hexadecanoic acid in IUPAC nomenclature, is the most common saturated fatty acid found in animals, plants and microorganisms. v Its chemical formula is CH 3(CH 2)14 COOH, and its C: D is 16: 0. As its name indicates, it is a major component of the oil from the fruit of oil palms (palm oil). v Palmitic acid can also be found in meats, cheeses, butter, and dairy products. v Palmitates are the salts and esters of palmitic acid. v The palmitate anion is the observed form of palmitic acid at physiologic p. H (7. 4). v Aluminum salts of palmitic acid and naphthenic acid were combined during World War II to produce napalm. v The word "napalm" is derived from the words naphthenic acid and palmitic acid.

Occurrence and production v Palmitic acid was discovered by Edmond Frémy in 1840, in saponified palm oil. v This remains the primary industrial route for its production, with the triglycerides (fats) in palm oil being hydrolysed by high temperature water (above 200 °C or 390 °F), and the resulting mixture fractionally distilled to give the pure product. v Palmitic acid is naturally produced by a wide range of other plants and organisms, typically at low levels. v It is naturally present in butter, cheese, milk, and meat, as well as cocoa butter, soybean oil, and sunflower oil. v Karukas contain 44. 90% palmitic acid. The cetyl ester of palmitic acid (cetyl palmitate) occurs in spermaceti.

Biochemistry v v Excess carbohydrates in the body are converted to palmitic acid. Palmitic acid is the first fatty acid produced during fatty acid synthesis and is the precursor to longer fatty acids. As a consequence, palmitic acid is a major body component of animals. In humans, one analysis found it to make up 21– 30% (molar) of human depot fat, and it is a major, but highly variable, lipid component of human breast milk. v Palmitate negatively feeds back on acetyl-Co. A carboxylase (ACC), which is responsible for converting acetyl. Co. A to malonyl-Co. A, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. v In biology, some proteins are modified by the addition of a palmitoyl group in a process known as palmitoylation. v Palmitoylation is important for membrane localisation of many proteins.

Applications v v v Palmitic acid is used to produce a soaps, cosmetics, and industrial mold release agents. These applications use sodium palmitate, which is commonly obtained by saponification of palm oil. To this end, palm oil, rendered from palm tree (species Elaeis gu ineensis), is treated with sodium hydroxide (in the form of caustic soda or lye), which causes hydrolysis of the ester groups, yielding glycerol and sodium palmitate. v Because it is inexpensive and adds texture and "mouth feel" to processed foods (convenience food), palmitic acid and its sodium salt find wide use in foodstuffs. v Sodium palmitate is permitted as a natural additive in organic products. v The aluminum salt is used as a thickening agent of napalm used in military actions. v Hydrogenation of palmitic acid yields cetyl alcohol, which is used to produce detergents and cosmetics.

v Recently, a long-acting antipsychotic medication, paliperidone palmitate (marketed as INVEGA Sustenna), used in the treatment of schizophrenia, has been synthesized using the oily palmitate ester as a long-acting release carrier medium when injected intramuscularly. v The underlying method of drug delivery is similar to that used with decanoic acid to deliver long-acting depot medication, in particular, neuroleptics such as haloperidol deaconate. Health effects v According to the World Health Organization, evidence is "convincing" that consumption of palmitic acid increases the risk of developing cardiovascular disease, based on studies indicating that it may increase LDL levels in the blood. v Retinyl palmitate is a source of vitamin A added to low fat milk to replace the vitamin content lost through the removal of milk fat. v Palmitate is attached to the alcohol form of vitamin A, retinol, to make vitamin A stable in milk.


 Promotion from assistant to associate professor
Promotion from assistant to associate professor Cuhk salary scale 2020
Cuhk salary scale 2020 Good morning val
Good morning val Mercy regional medical center lorain
Mercy regional medical center lorain 7 times a day i praise you
7 times a day i praise you Mercy and goodness give me assurance
Mercy and goodness give me assurance Unit 5 quality of mercy
Unit 5 quality of mercy U of d mercy school of dentistry
U of d mercy school of dentistry Your mercy flows like a river wide
Your mercy flows like a river wide The kingdom of god does not come with observation
The kingdom of god does not come with observation Come lord jesus come and be born in our hearts lyrics
Come lord jesus come and be born in our hearts lyrics Mercy degreeworks
Mercy degreeworks Blessed are the merciful for they will be shown mercy
Blessed are the merciful for they will be shown mercy Mercy college adjunct positions
Mercy college adjunct positions The crucible main characters
The crucible main characters Matthew 5 10 esv
Matthew 5 10 esv There's a place where mercy reigns
There's a place where mercy reigns What is mercy killing
What is mercy killing Lamb of god you take away the sin of the world
Lamb of god you take away the sin of the world Mercy seat bible
Mercy seat bible War without mercy summary
War without mercy summary Dr bolyard
Dr bolyard Ramadan month of mercy
Ramadan month of mercy Character
Character God you are good and your mercy endureth forever
God you are good and your mercy endureth forever Like a rushing wind jesus breathe within
Like a rushing wind jesus breathe within
















































