Do Now and Announcements Do Now Work on






- Slides: 18

Do Now and Announcements �Do Now: Work on Orbital Diagram questions �Unit 4 Test WED 11/19, THURS 11/20 ◦ Review Key is posted online

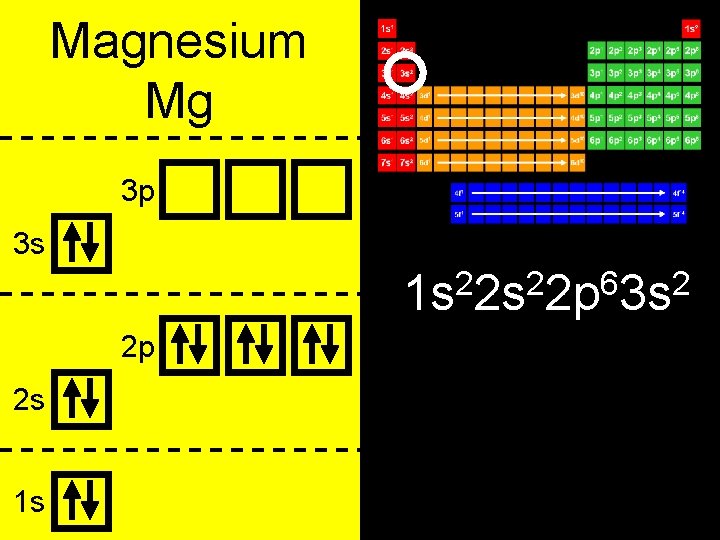
Magnesium Mg 3 p 3 s 2 2 6 2 1 s 2 s 2 p 3 s 2 p 2 s 1 s

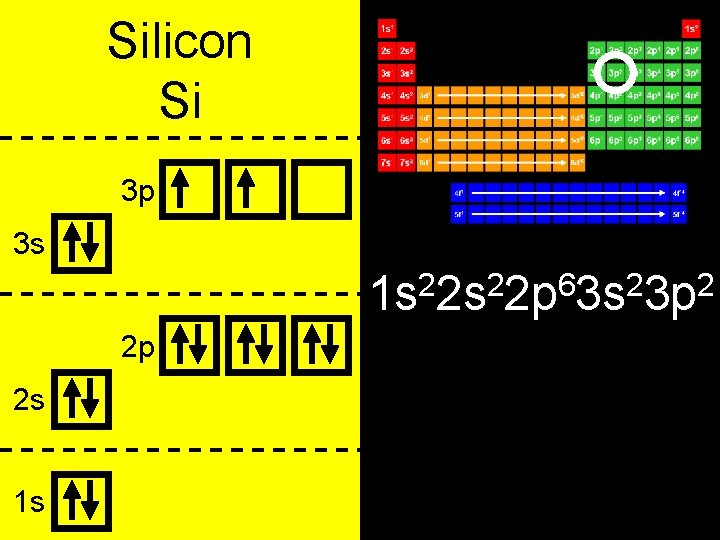
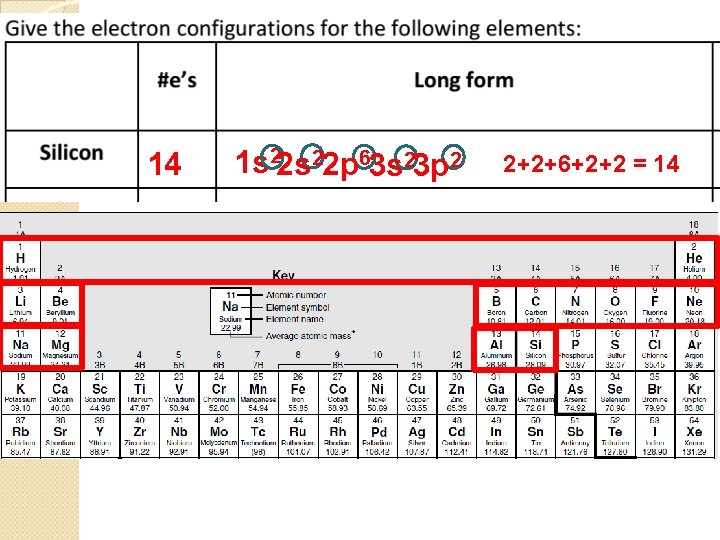
Silicon Si 3 p 3 s 2 2 6 2 2 1 s 2 s 2 p 3 s 3 p 2 p 2 s 1 s

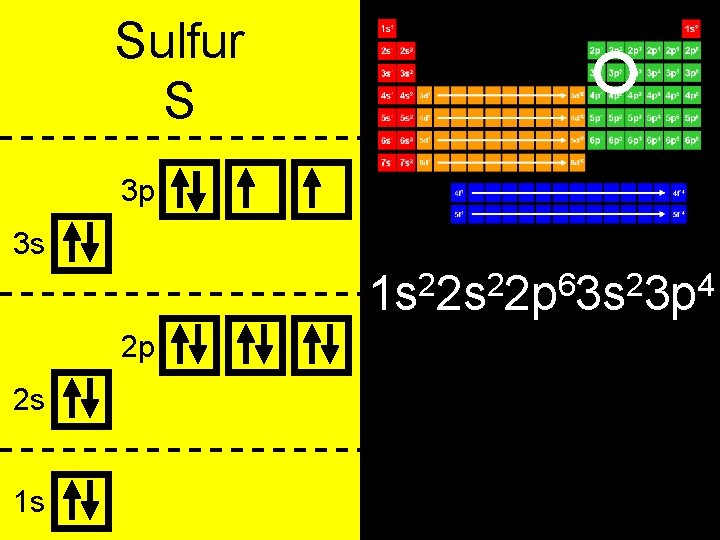
Sulfur S 3 p 3 s 2 2 6 2 4 1 s 2 s 2 p 3 s 3 p 2 p 2 s 1 s

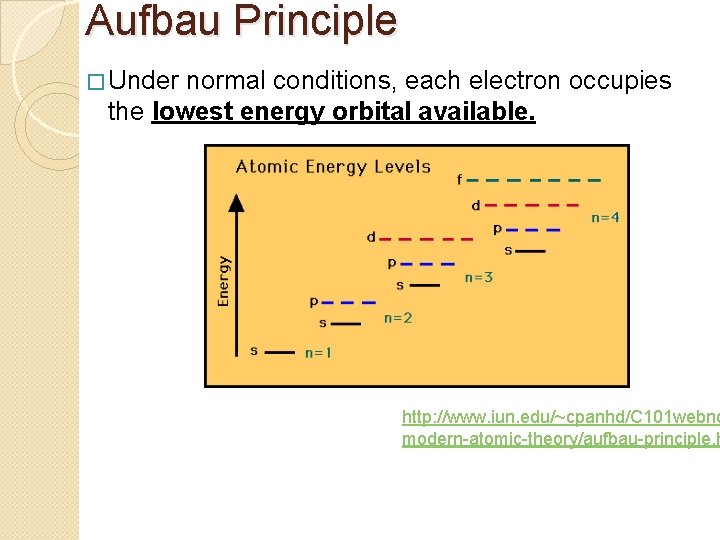
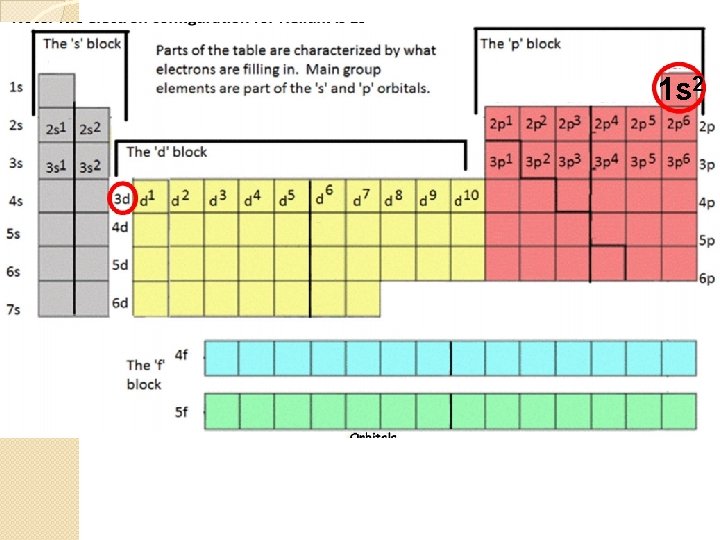
Aufbau Principle � Under normal conditions, each electron occupies the lowest energy orbital available. http: //www. iun. edu/~cpanhd/C 101 webno modern-atomic-theory/aufbau-principle. h

Pauli Exclusion Principle � Every electron spins; it can spin in one of two directions. ◦ An arrow pointing up (↑) indicates one direction. ◦ An arrow pointing down (↓) indicates the other direction. � The Pauli exclusion principle states that a maximum of two electrons (an electron pair) can occupy an orbital space, but only if they have opposing spins. OR RIGHT WRONG

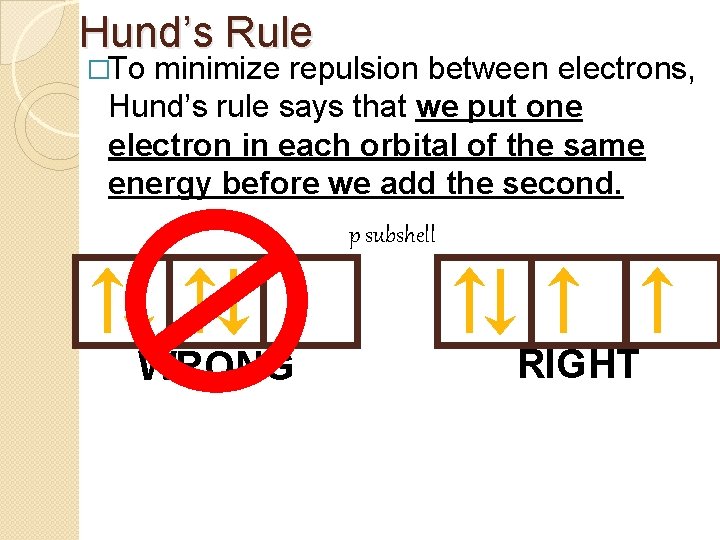
Hund’s Rule �To minimize repulsion between electrons, Hund’s rule says that we put one electron in each orbital of the same energy before we add the second. p subshell WRONG RIGHT

1 s 2

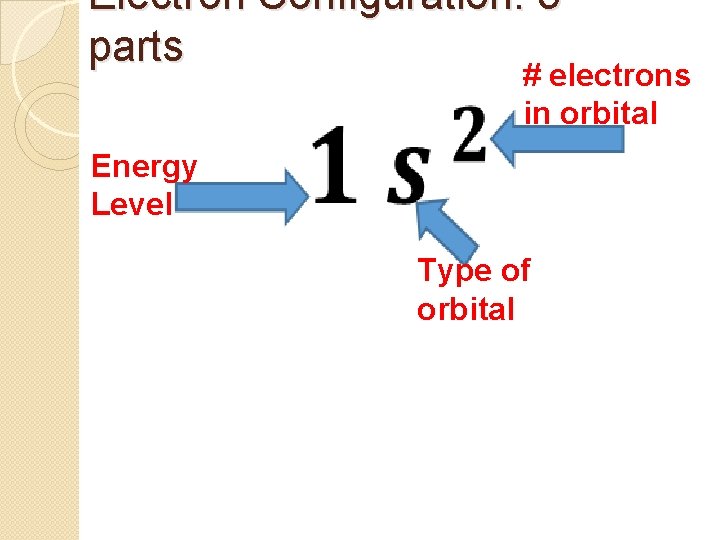
Electron Configuration: 3 parts # electrons in orbital Energy Level Type of orbital

14 1 s 22 p 63 s 23 p 2 2+2+6+2+2 = 14

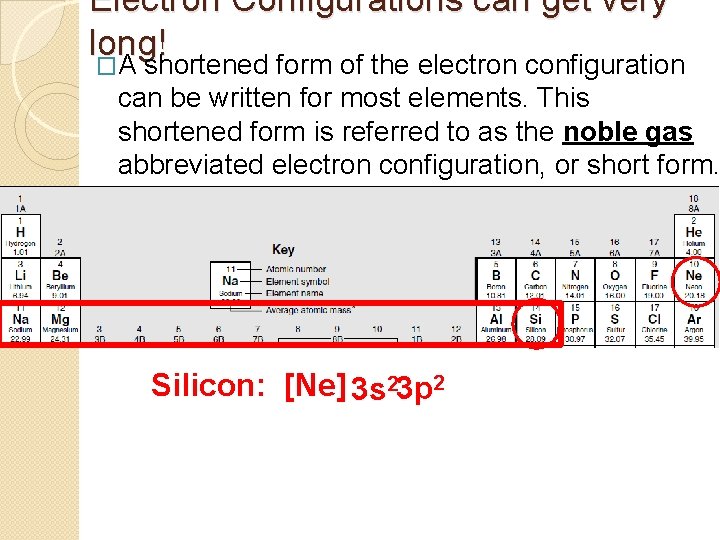
Electron Configurations can get very long! �A shortened form of the electron configuration can be written for most elements. This shortened form is referred to as the noble gas abbreviated electron configuration, or short form. Silicon: [Ne] 3 s 23 p 2

Valence Electrons �The electrons in the outermost shell or highest energy level �Total number of electrons in the highest energy s and p orbitals!

Electronic Battleship

Noble Gases: Group 18 �Have octet=full valence (outermost) shell ◦ All elements want to be a noble gas (so freaking bad). In other words, all elements want to have a full valence shell ◦ Remember: “ 8 IS GREAT!” ◦ Exception: helium: it only has 2 valence electrons

Obtaining a Noble Gas Electron Configuration �Elements can lose or gain electrons in order to get a noble gas’ electron configuration ◦ Depending on the element, sometimes it is “easier to lose electron(s)”, and sometimes it is “easier to gain electron(s)”

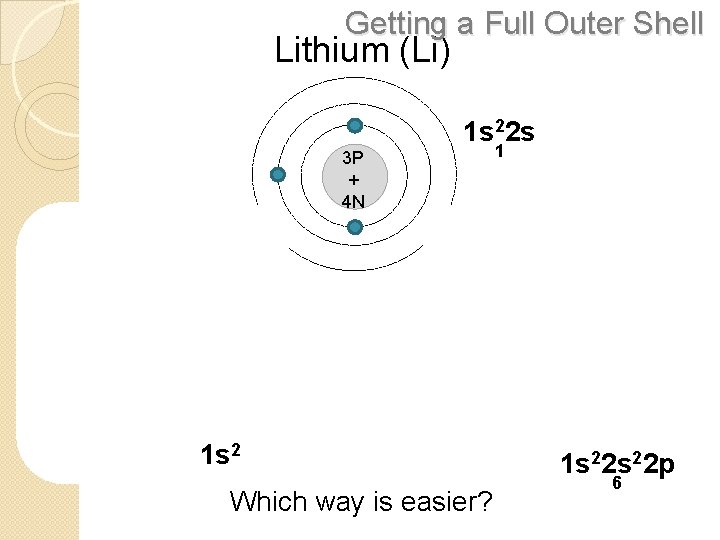
Getting a Full Outer Shell Lithium (Li) 1 s 22 s 3 P + 4 N REMOVE 1 ELECTRON 3 P + 4 N 1 ADD 7 ELECTRONS 3 P + 4 N 1 s 2 Which way is easier? 1 s 22 p 6

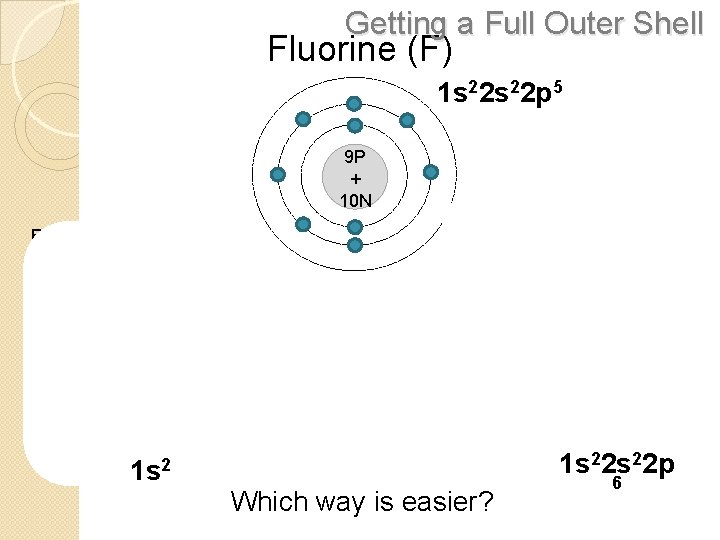
Getting a Full Outer Shell Fluorine (F) 1 s 22 p 5 9 P + 10 N ADD 1 ELECTRON REMOVE 7 ELECTRONS 9 P + 10 N 1 s 22 s 22 p Which way is easier? 6
