Development of a CSTD testing protocol Experimental Evidence







- Slides: 18

Development of a CSTD testing protocol • Experimental Evidence for Selection of CSTD Challenge Compounds Amos Doepke and Robert P. Streicher NIOSH/DART/CEMB 11/07/2016

Disclaimer The findings and conclusions in this presentation have not been formally disseminated by the National Institute for Occupational Safety and Health and should not be construed to represent any agency determination or policy.

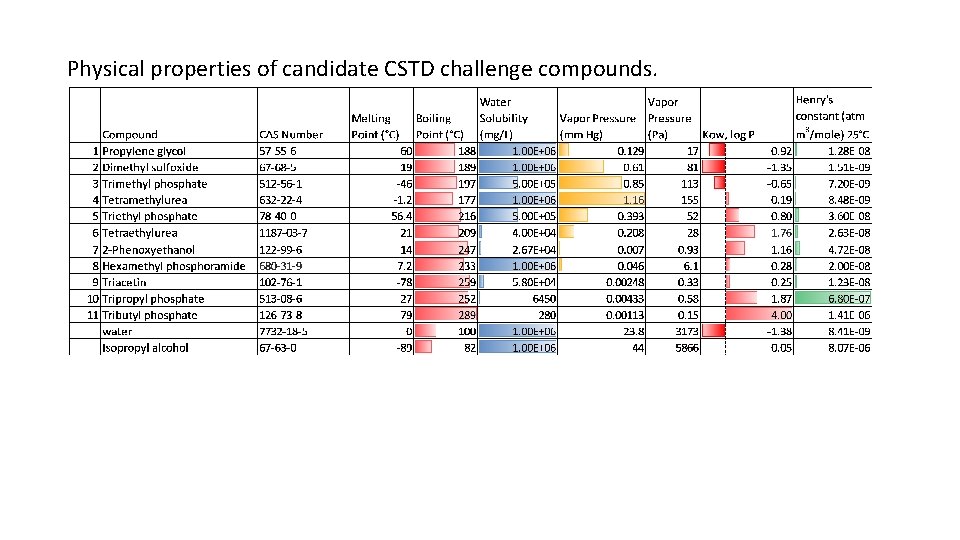
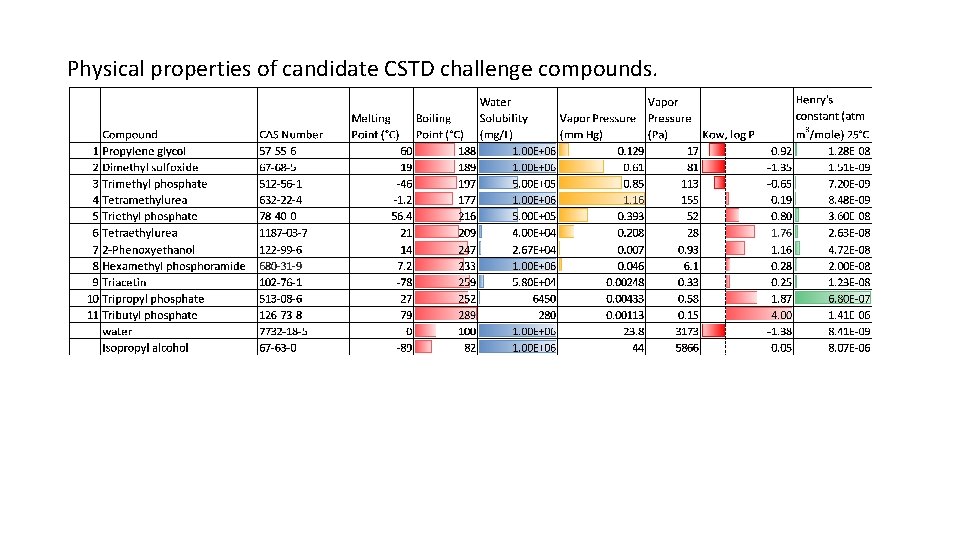
Considerations for Selection of CSTD Challenge Compounds • Use the most volatile drug on NIOSH list as reference: thiotepa, vapor pressure: 9 x 10 -3 mm Hg @ 25°C (EST, EPA EPISuite, accessed 2016) • Consider compounds of similar vapor pressures, up to about 100 x, i. e. 1 x 10 -2 mm Hg to 1 mm Hg • Compounds must have minimum water solubility of at least 0. 1% • Compounds should be liquid at room temperature to facilitate evaporation and subsequent detection • Consider compounds of structural similarity to hazardous drugs if convenient. With two of the most volatile agents being thiotepa (a thiophosphamide) and hydroxyurea (a urea), trialkyl phosphates and tetraalkyl ureas are candidates. 2 Phenoxyethanol was suggested by HSE. Several semi-volatile solvents are candidates. Photo: NIOSH

Physical properties of candidate CSTD challenge compounds.

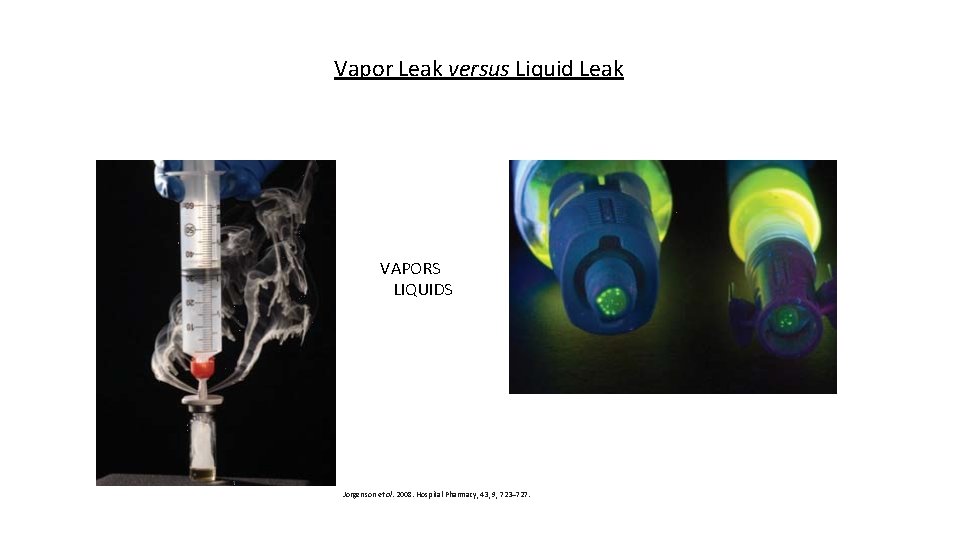
Vapor Leak versus Liquid Leak VAPORS LIQUIDS Jorgenson et al. 2008. Hospital Pharmacy, 43, 9, 723– 727.

Vapor Leak versus Liquid Leak • Potential for multiple routes of material release • Challenge compounds can enable differentiation of vapor and liquid leaks • Distinguishing a vapor leak and liquid leak may be diagnostic • Vapor leaks and liquid leaks may represent very different levels of concern Jorgenson et al. 2008. Hospital Pharmacy, 43, 9, 723– 727.

Physical properties of candidate CSTD challenge compounds.

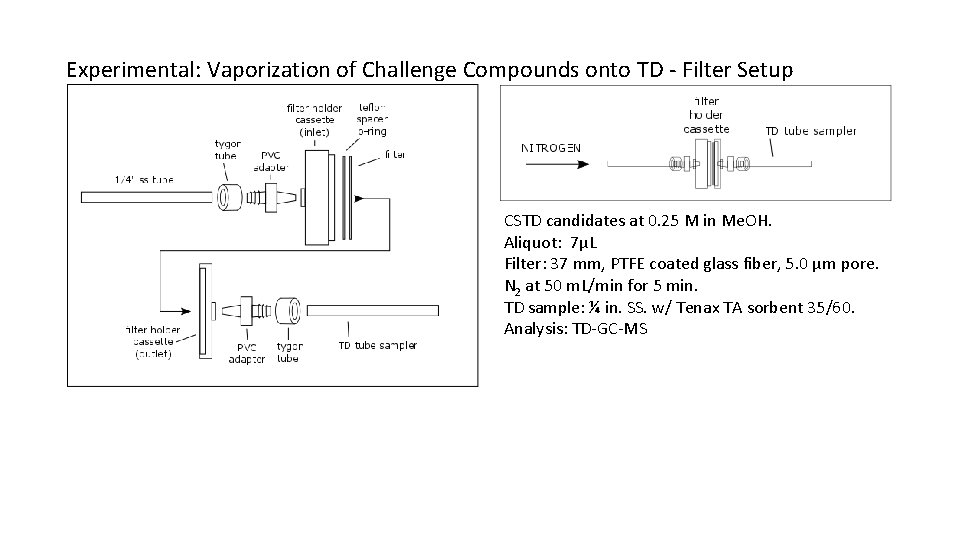
Experimental: Vaporization of Challenge Compounds onto TD - Filter Setup CSTD candidates at 0. 25 M in Me. OH. Aliquot: 7µL Filter: 37 mm, PTFE coated glass fiber, 5. 0 µm pore. N 2 at 50 m. L/min for 5 min. TD sample: ¼ in. SS. w/ Tenax TA sorbent 35/60. Analysis: TD-GC-MS

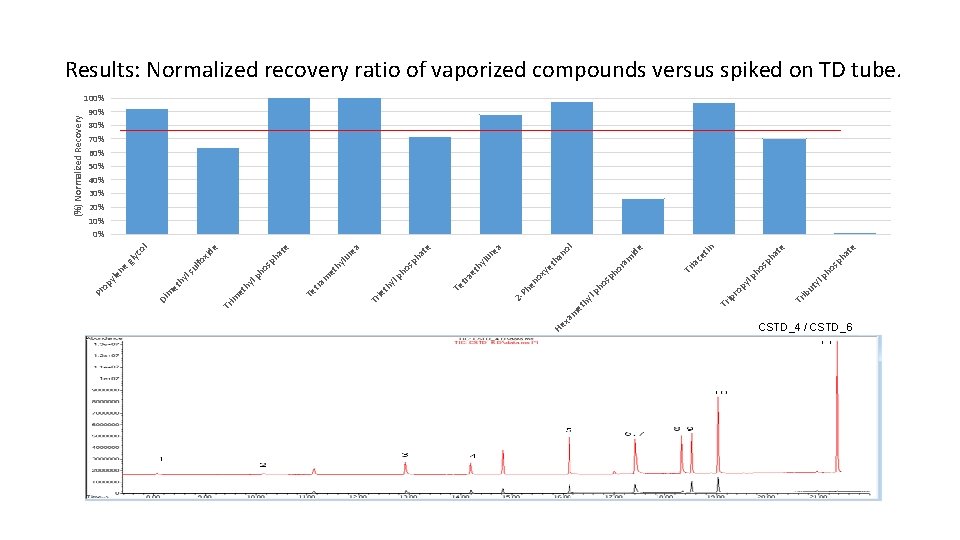
te ha sp sp ho ho lp lp ty ib u Tr py ro ce tin Tr ia id e m ra ho sp l no lu re a et ha xy no ho lp Tr ip hy et xa m He Ph e 2 - hy et e at ph os ph ra Te t yl th Tr ie a re te ha lu hy et m sp ho e xid l co gly fo ul ls lp tra Te hy et im Tr hy et Di m ne le py Pr o (%) Normalized Recovery Results: Normalized recovery ratio of vaporized compounds versus spiked on TD tube. 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% CSTD_4 / CSTD_6

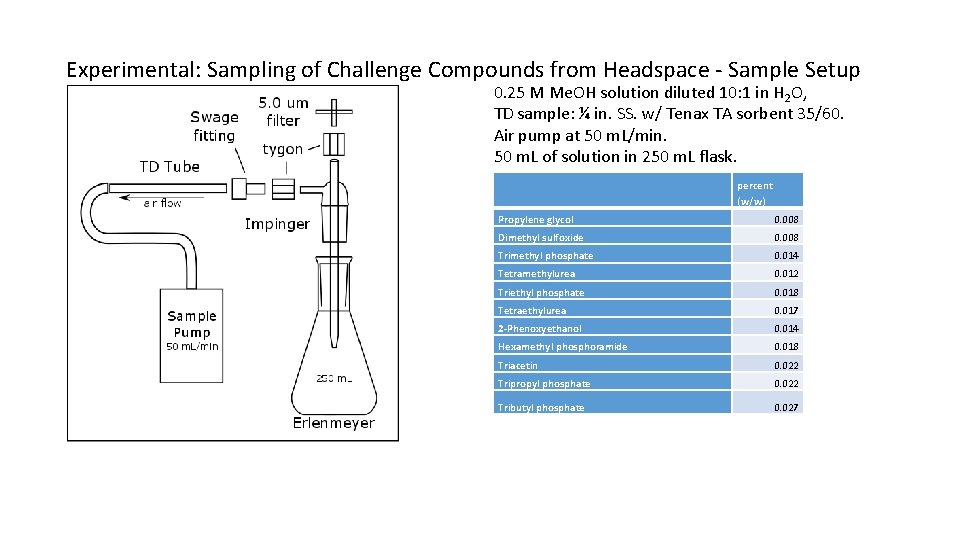
Experimental: Sampling of Challenge Compounds from Headspace - Sample Setup 0. 25 M Me. OH solution diluted 10: 1 in H 2 O, TD sample: ¼ in. SS. w/ Tenax TA sorbent 35/60. Air pump at 50 m. L/min. 50 m. L of solution in 250 m. L flask. percent (w/w) Propylene glycol 0. 008 Dimethyl sulfoxide 0. 008 Trimethyl phosphate 0. 014 Tetramethylurea 0. 012 Triethyl phosphate 0. 018 Tetraethylurea 0. 017 2 -Phenoxyethanol 0. 014 Hexamethyl phosphoramide 0. 018 Triacetin 0. 022 Tripropyl phosphate 0. 022 Tributyl phosphate 0. 027

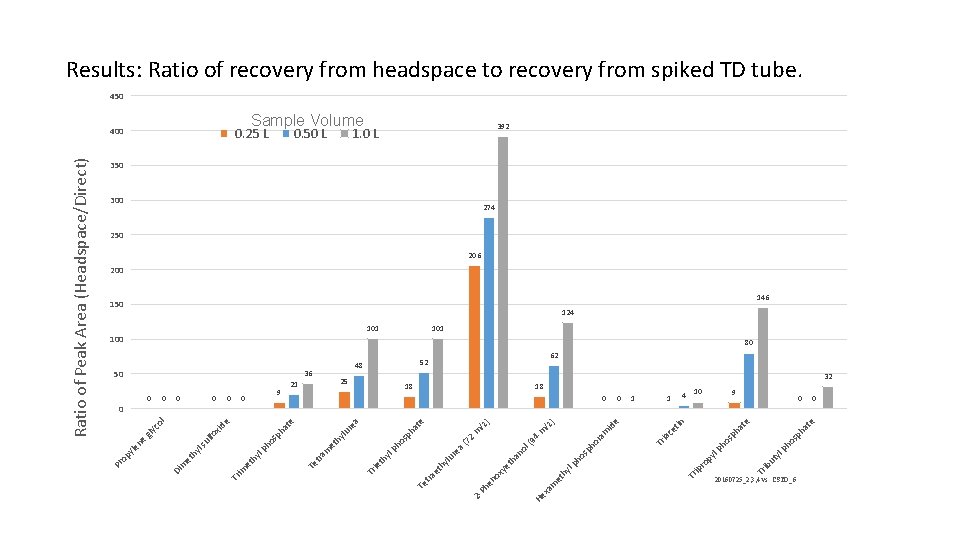
e at ph os ph 10 yl 100 ut Tr ib e 4 sp ha t ho lp py 1 ro 1 ip in 18 Tr ce t 0 ia 0 Tr 150 e ) /z id m 52 am or 94 18 ho sp h lp hy et m xa ) /z 1. 0 L He m 300 ol ( an th (7 2 48 ye ea lu r te 101 2 Ph en ox hy et tra ph a os ph 25 Te yl 21 th 36 ie a Sample Volume 0. 50 L Tr lu re 9 hy 0 et 50 m 0. 25 L tra te 0 ha sp lp ho 400 Te hy et 0 im id e 0 Tr fo x ul ls hy et 0 m ol gly c 0 0 Di ne yle op Pr Ratio of Peak Area (Headspace/Direct) Results: Ratio of recovery from headspace to recovery from spiked TD tube. 450 392 350 274 250 206 200 146 124 101 62 80 32 9 0 20160725_2, 3, 4 vs. CSTD_6 0

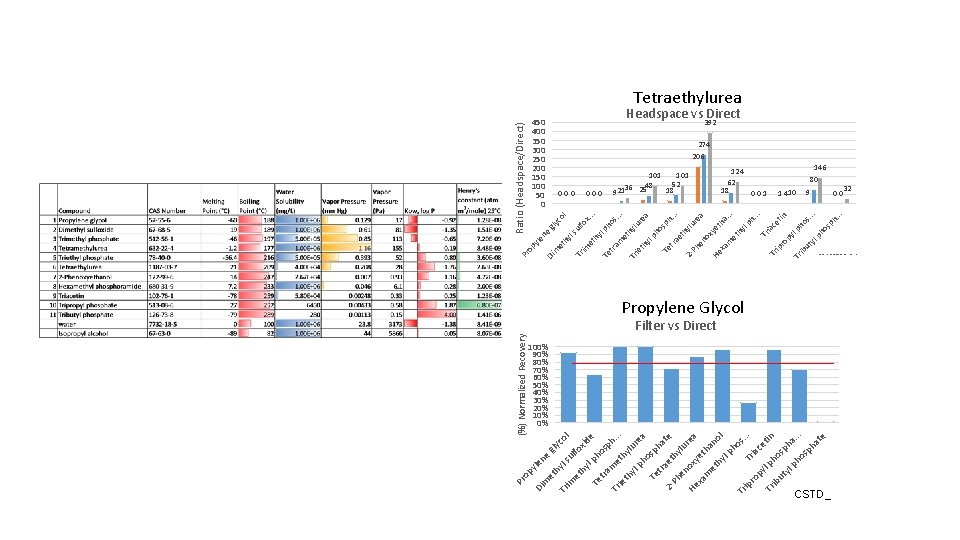
ho lp sp e xid l m h. Tr et. ie hy. th l u yl ph rea o sp Te tra ha te 2 et Ph hy en lu He oxy rea et xa ha m et no hy lp l ho Tr s. . ip ro Tria. py c l p etin Tr ho ib ut sp yl ha ph. os. . ph at e tra hy co ib yl ut . . . os ph s. . . 1 410 ph . . in ce t ho lp py ro ia h. 124 62 18 001 Tr ip Tr Tr lp . . . th a a 101 52 48 36 25 18 921 hy m et ye . . . a re lu sp h hy ox en xa He Ph 2 - ho re hy lu tra et Te lp th y ie s. . 000 Tr ho lp m et ra Te t l x. . fo ls ul hy et Tr im gly co Ratio (Headspace/Direct) ne yle et hy m Di op Pr 000 Te fo ul ls gly (%) Normalized Recovery 450 400 350 300 250 200 150 100 50 0 et Tr im hy ne le py et Di m Pr o Tetraethylurea Headspace vs Direct 392 206 274 146 80 9 00 Propylene Glycol Filter vs Direct 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% CSTD_ 32 20160725_2, 3, 4 vs. CSTD_6

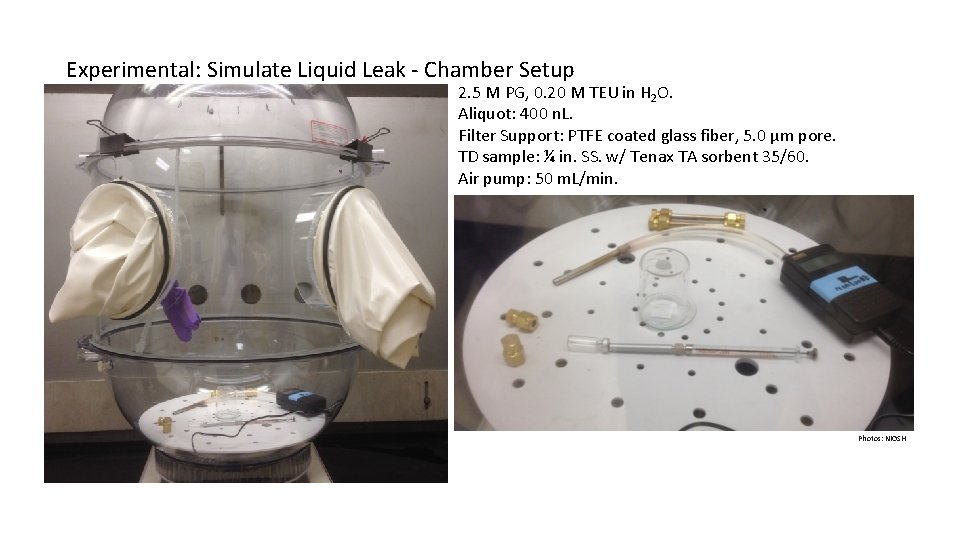
Experimental: Simulate Liquid Leak - Chamber Setup 2. 5 M PG, 0. 20 M TEU in H 2 O. Aliquot: 400 n. L. Filter Support: PTFE coated glass fiber, 5. 0 µm pore. TD sample: ¼ in. SS. w/ Tenax TA sorbent 35/60. Air pump: 50 m. L/min. Photos: NIOSH

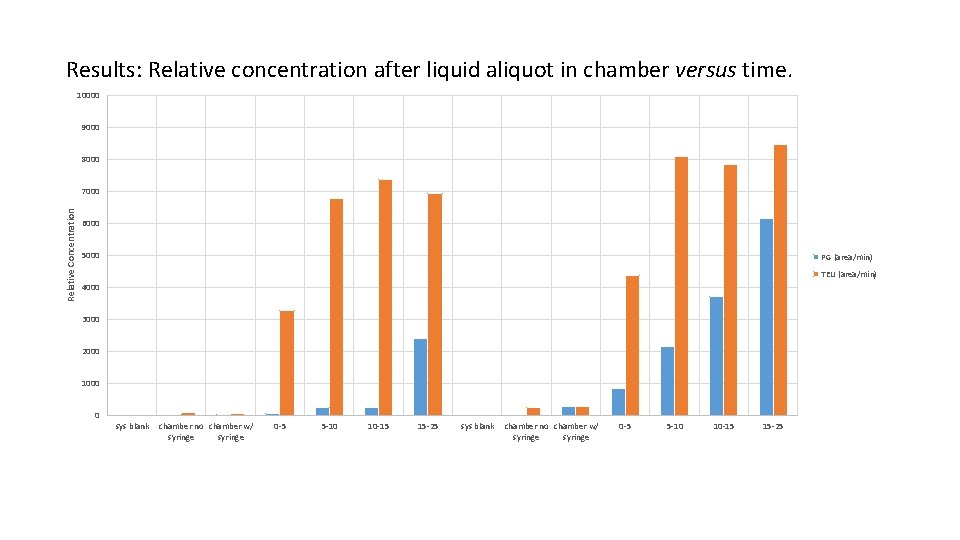
Results: Relative concentration after liquid aliquot in chamber versus time. 10000 9000 8000 Relative Concentration 7000 6000 5000 PG (area/min) TEU (area/min) 4000 3000 2000 1000 0 sys blank chamber no chamber w/ syringe 0 -5 5 -10 10 -15 15 -25

Is there an ideal challenge compound mixture? Quantitative Diagnostic Photos: Left: top and bottom, Jorgenson et al. , Right : NIOSH

The next steps for the CSTD testing protocol • Gather feedback from scientific community and stakeholders. • Consider multiple variables (mixture components, concentrations, material interactions, time, temperature, humidity) • Continue to develop analysis methods. (Protocols, LOQ, LOD) • Thermal Desorption Tube, TD-GC-MS • Low Detection Limits, Ability to Concentrate Compounds • Selected-Ion Flow-Tube Mass Spectrometry, SIFT-MS • Real time detection / quantification • No sample handling • LOQs comparable to TD-GC-MS • Expensive ~$250 K • Challenge CSTD components to testing. • Validate a comprehensive testing protocol.

Thank you for your contributions to this research • • • Gayle De. Bord – NOSH/DART/OD Ronald Hall, Kenneth Mead and Deborah Hirst – NIOSH/DART/EPHB Lee Greenawald – NIOSH/NPPTL Thomas H. Connor – NIOSH/DART/EPHB Stakeholders who have shared their expertise

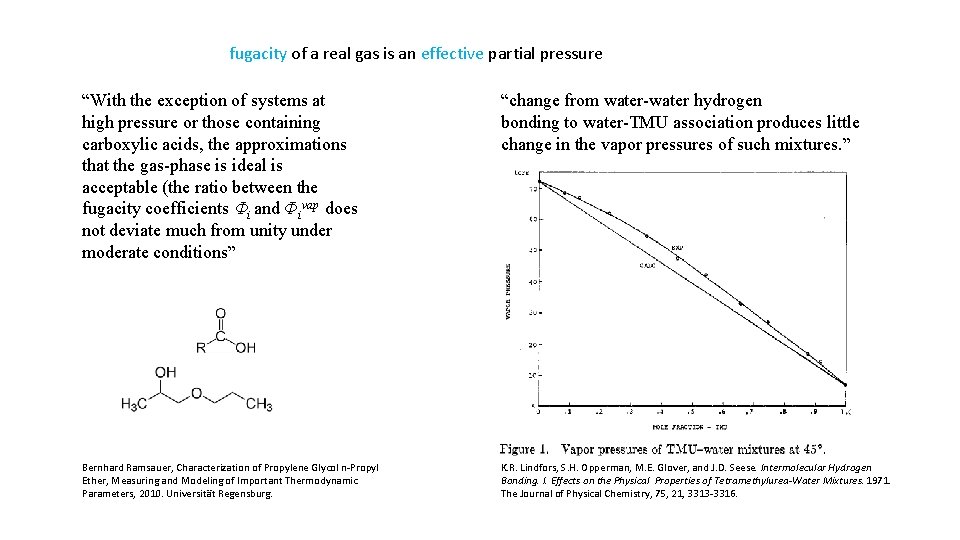
fugacity of a real gas is an effective partial pressure “With the exception of systems at high pressure or those containing carboxylic acids, the approximations that the gas-phase is ideal is acceptable (the ratio between the fugacity coefficients Φi and Фivap does not deviate much from unity under moderate conditions” “change from water-water hydrogen bonding to water-TMU association produces little change in the vapor pressures of such mixtures. ” Bernhard Ramsauer, Characterization of Propylene Glycol n-Propyl Ether, Measuring and Modeling of Important Thermodynamic Parameters, 2010. Universität Regensburg. K. R. Lindfors, S. H. Opperman, M. E. Glover, and J. D. Seese. Intermolecular Hydrogen Bonding. I. Effects on the Physical Properties of Tetramethylurea-Water Mixtures. 1971. The Journal of Physical Chemistry, 75, 21, 3313 -3316.