CUNY Human Research Protection Program HRPP School of









- Slides: 14

CUNY Human Research Protection Program (HRPP) School of Professional Studies April 18, 2013 http: //www. cuny. edu/research/compliance/human-subjects-research-1. html

CUNY HRPP - Responsibilities • Ensure protection of human subjects in research • Ensure compliance with federal regulations, State law & CUNY policies

CUNY HRPP - Structure • College specific HRPP Coordinators at most Colleges • 2 IRB Administrators • 4 Convened IRB Panels • 1 Expedited Review Panel

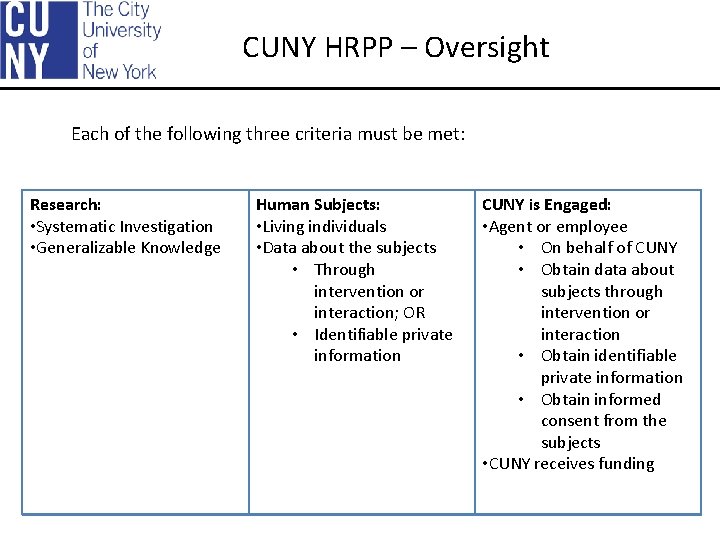
CUNY HRPP – Oversight Each of the following three criteria must be met: Research: • Systematic Investigation • Generalizable Knowledge Human Subjects: • Living individuals • Data about the subjects • Through intervention or interaction; OR • Identifiable private information CUNY is Engaged: • Agent or employee • On behalf of CUNY • Obtain data about subjects through intervention or interaction • Obtain identifiable private information • Obtain informed consent from the subjects • CUNY receives funding

CUNY HRPP – Is Review Required? • Class assignments / program evaluation / SOTL – Yes: IF intended for generalizable knowledge • Publications • Conference presentations • Making available in the public domain – No: IF data to be presented within the confines of the institution

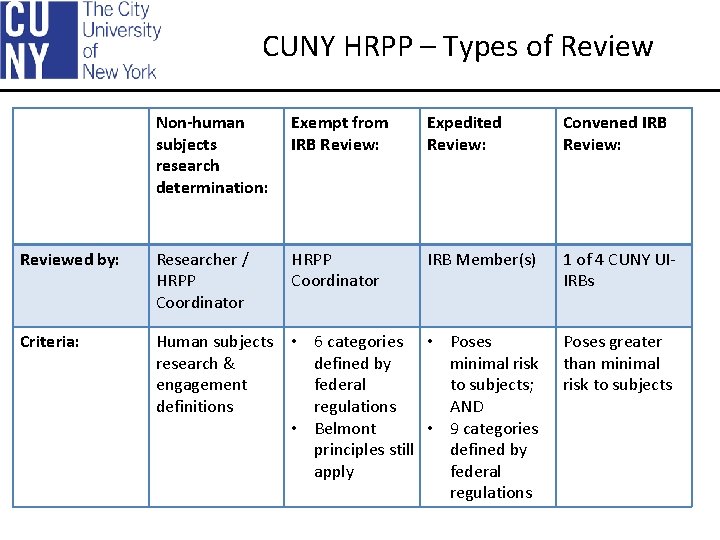
CUNY HRPP – Types of Review Non-human subjects research determination: Exempt from IRB Review: Expedited Review: Convened IRB Review: Reviewed by: Researcher / HRPP Coordinator IRB Member(s) 1 of 4 CUNY UIIRBs Criteria: Human subjects research & engagement definitions • 6 categories • Poses defined by minimal risk federal to subjects; regulations AND • Belmont • 9 categories principles still defined by apply federal regulations Poses greater than minimal risk to subjects

CUNY HRPP - Submission • Submission Process: – IRB Net: https: //www. irbnet. org – New User Registration – Submit to CUNY Central Office HRPP • Guidance: – Arita Winter, CUNY Central Office HRPP Coordinator • arita. winter@cuny. edu; 646 -664 -8919

CUNY HRPP - Submission • Application Part I = basic information – Required for all studies • Request for Exemption • Application Part II – Required for non-exempt human subjects research – For review via expedited review procedures – For review by convened IRB • Various Supplements

CUNY IRB – Criteria for Approval • Experimental Design and Scientific Validity – Ensure that risks are minimized • Risk / Benefit Analysis – Minimize risks • Examples of safeguards: 1) adequate training of research personnel; 2) exclusion of populations at increased risk; 3) adequate data protection; 4) adequate safety monitoring – Ensure risks are reasonable in relation to benefits – Types of risks: physical; psychological; social; legal; economic; invasion of privacy; breach of confidentiality

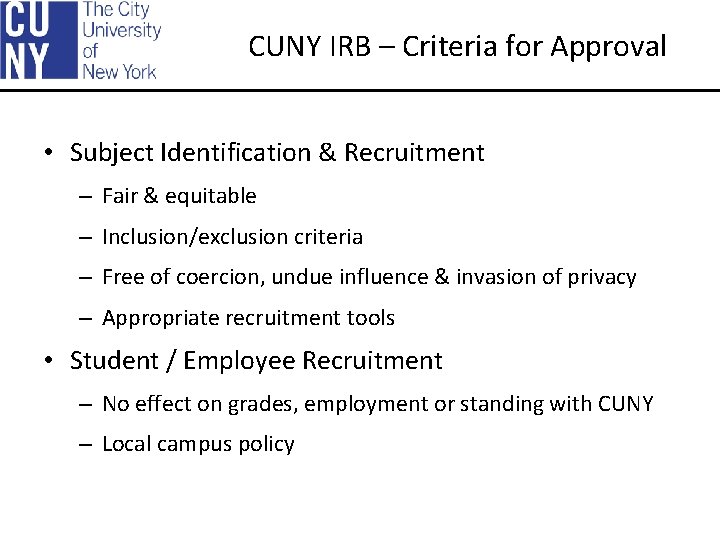
CUNY IRB – Criteria for Approval • Subject Identification & Recruitment – Fair & equitable – Inclusion/exclusion criteria – Free of coercion, undue influence & invasion of privacy – Appropriate recruitment tools • Student / Employee Recruitment – No effect on grades, employment or standing with CUNY – Local campus policy

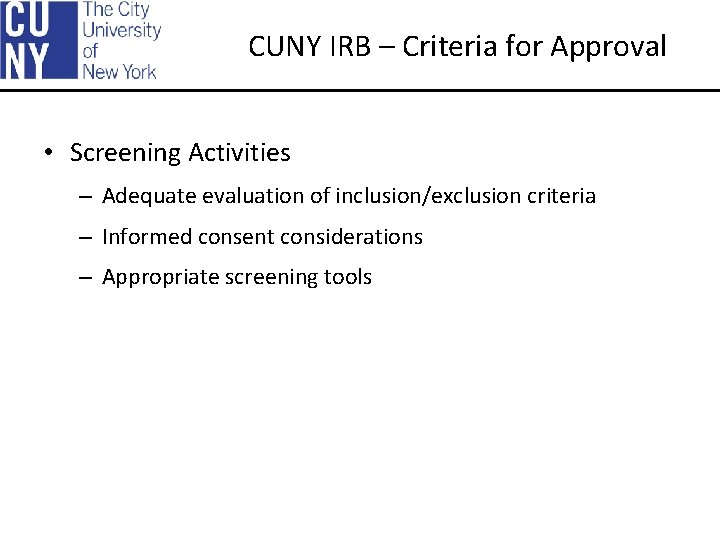
CUNY IRB – Criteria for Approval • Screening Activities – Adequate evaluation of inclusion/exclusion criteria – Informed consent considerations – Appropriate screening tools

CUNY IRB – Criteria for Approval • Informed Consent Process & Documentation – Process takes place in a manner & at location that ensure privacy – Information is provided in a manner & language understood by subject – Sufficient opportunity to consider participation – Subject’s questions are answered – Voluntary consent – Voluntary withdrawal without penalty – Individual obtaining consent is qualified to do so – Legal rights – no exculpatory language

CUNY IRB – Criteria for Approval • Waivers of informed consent – Waiver of documented informed consent – Waiver of informed consent – Alteration of informed consent • Conflict of Interest – Existing or potential financial conflict – Conflict of commitment

Faculty Advisor Responsibilities • Overall project – Ensure feasibility of project – Ensure that there are sufficient resources to complete the project – Ensure that student is adequately trained to conduct the project • Review of HRPP/IRB submission – Ensure completeness & accuracy – Ensure consistencies across documents – Check language & grammar – Ensure compliance with CUNY policies, applicable regulations & sponsor requirements