CSTE 2012 National Electronic Disease Surveillance System NEDSS





![Other Innovations • “[State] is utilizing the RCMT, labs recorded in the NBS, laboratory Other Innovations • “[State] is utilizing the RCMT, labs recorded in the NBS, laboratory](https://slidetodoc.com/presentation_image_h2/4f803e0d5a1fdd67a7aace8a992846f9/image-19.jpg)


- Slides: 21

CSTE 2012 National Electronic Disease Surveillance System (NEDSS) Assessment CSTE Annual Conference – Pasadena , CA Erin Holt, MPH – TN Kathryn Turner, Ph. D – ID

Background • August 2007: CSTE conducted a national assessment to evaluate status and progress states had made in the realm of electronic disease surveillance (http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 5829 a 3. htm) • February 2010: CSTE administered a more in-depth assessment to collect additional information including progress, challenges, and obstacles to implementation (http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6041 a 3. htm) • August 2012: CSTE administered a follow-up assessment to the 2010 assessment to refine subject areas and address emerging topics

Methods • Assessment developed by ad hoc working group under CSTE Electronic Laboratory and Disease Reporting Subcommittee • Multiple choice questions with verbatim “Other” responses focused on system functionality, operations, and resources • Distributed via Survey. Monkey© • NEDSS Project Coordinators • State Epidemiologists • Responses received from all 50 states and D. C.

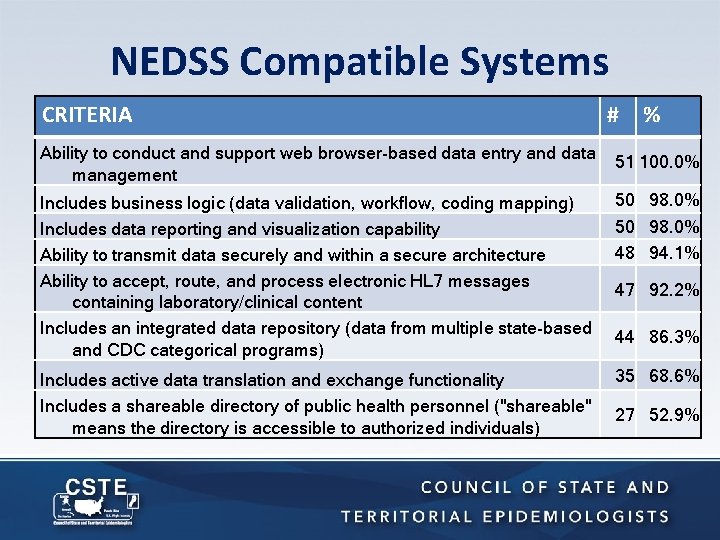
NEDSS Compatible Systems CRITERIA Ability to conduct and support web browser-based data entry and data management # % 51 100. 0% Includes business logic (data validation, workflow, coding mapping) Includes data reporting and visualization capability Ability to transmit data securely and within a secure architecture Ability to accept, route, and process electronic HL 7 messages containing laboratory/clinical content Includes an integrated data repository (data from multiple state-based and CDC categorical programs) 50 98. 0% Includes active data translation and exchange functionality Includes a shareable directory of public health personnel ("shareable" means the directory is accessible to authorized individuals) 35 68. 6% 50 98. 0% 48 94. 1% 47 92. 2% 44 86. 3% 27 52. 9%

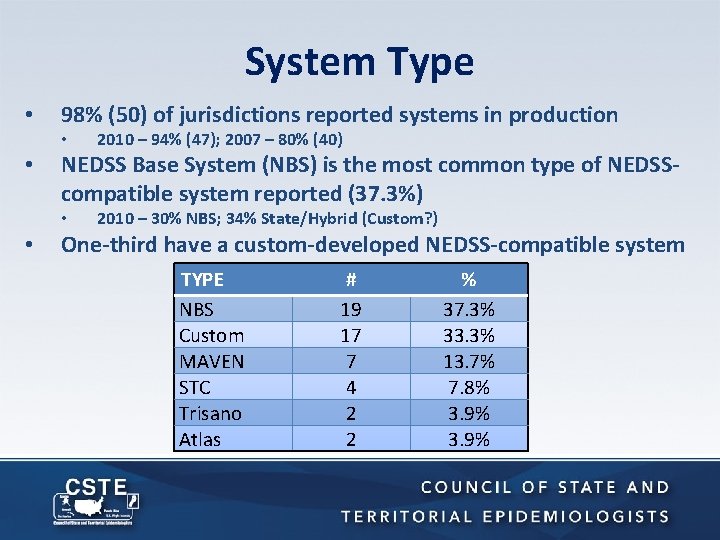
System Type • 98% (50) of jurisdictions reported systems in production • • NEDSS Base System (NBS) is the most common type of NEDSScompatible system reported (37. 3%) • • 2010 – 94% (47); 2007 – 80% (40) 2010 – 30% NBS; 34% State/Hybrid (Custom? ) One-third have a custom-developed NEDSS-compatible system TYPE NBS Custom MAVEN STC Trisano Atlas # 19 17 7 4 2 2 % 37. 3% 33. 3% 13. 7% 7. 8% 3. 9%

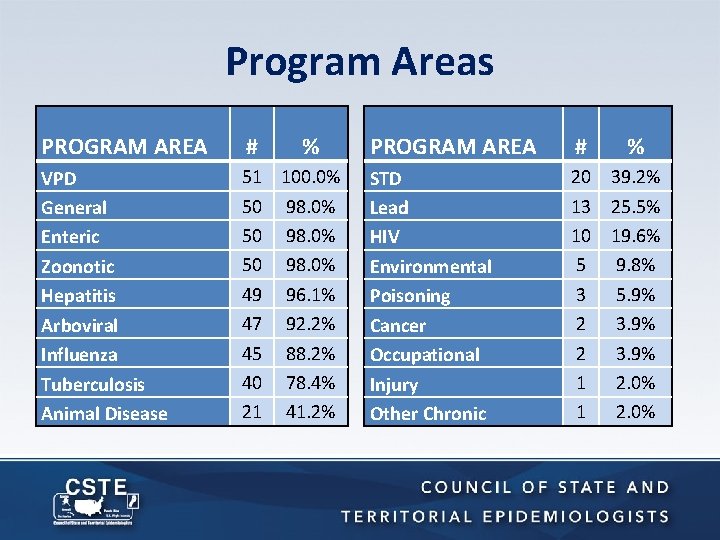
Program Areas PROGRAM AREA # % VPD General Enteric Zoonotic Hepatitis Arboviral Influenza Tuberculosis Animal Disease 51 100. 0% 50 98. 0% 49 96. 1% 47 92. 2% 45 88. 2% 40 78. 4% 21 41. 2% PROGRAM AREA # % STD Lead HIV Environmental Poisoning Cancer Occupational Injury Other Chronic 20 39. 2% 13 25. 5% 10 19. 6% 5 9. 8% 3 5. 9% 2 3. 9% 1 2. 0%

Surveillance System Functionality • 96% (n=49) could receive electronic lab reports • 2007 – 70% (28); 2010 - 90% (n=44) • 63% (n=32) could receive electronic case reports • 26 include web-entry for case reports • 35% (n=18) could receive, process, and store electronic case reports from another jurisdiction • 34% (n=17) report case management functionality • 46% (n=23) report contact tracing functionality • 26% (n=13) report outbreak management functionality

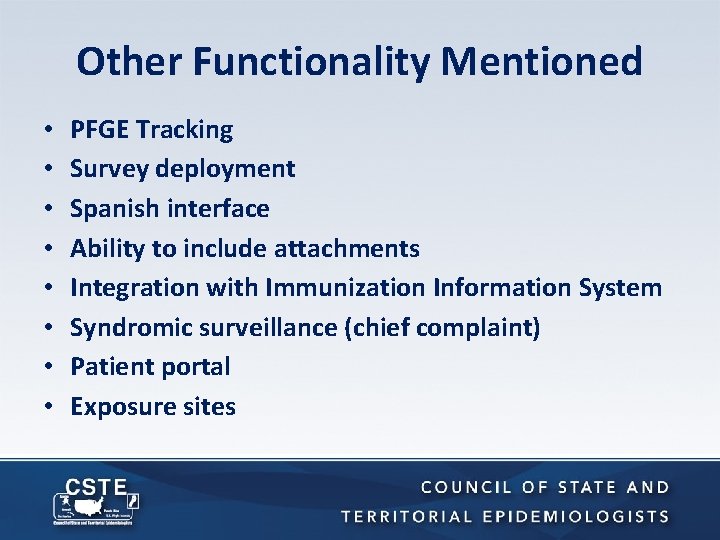
Other Functionality Mentioned • • PFGE Tracking Survey deployment Spanish interface Ability to include attachments Integration with Immunization Information System Syndromic surveillance (chief complaint) Patient portal Exposure sites

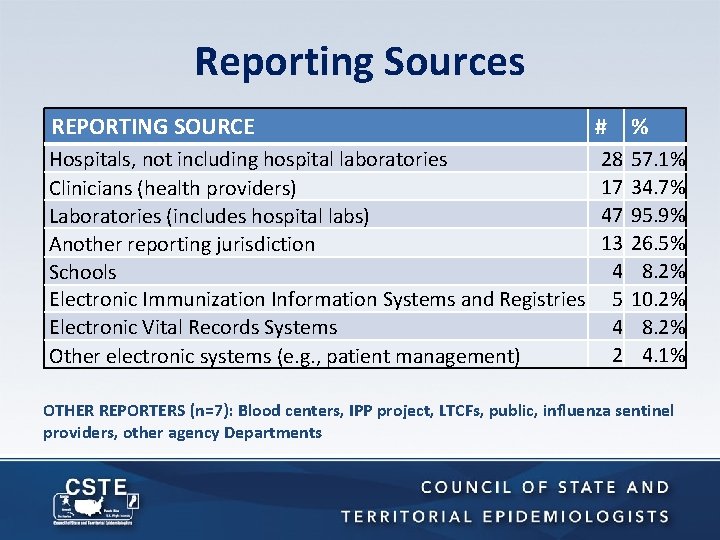
Reporting Sources REPORTING SOURCE # % Hospitals, not including hospital laboratories Clinicians (health providers) Laboratories (includes hospital labs) Another reporting jurisdiction Schools Electronic Immunization Information Systems and Registries Electronic Vital Records Systems Other electronic systems (e. g. , patient management) 28 17 47 13 4 5 4 2 57. 1% 34. 7% 95. 9% 26. 5% 8. 2% 10. 2% 8. 2% 4. 1% OTHER REPORTERS (n=7): Blood centers, IPP project, LTCFs, public, influenza sentinel providers, other agency Departments

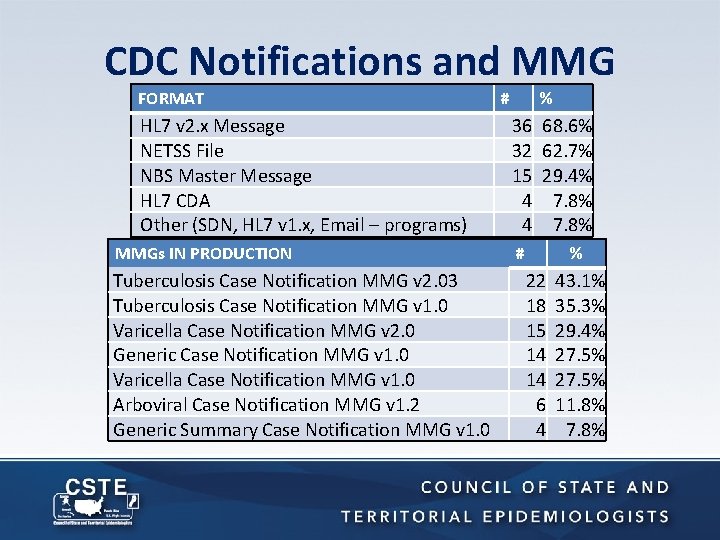
CDC Notifications and MMG FORMAT HL 7 v 2. x Message NETSS File NBS Master Message HL 7 CDA Other (SDN, HL 7 v 1. x, Email – programs) MMGs IN PRODUCTION Tuberculosis Case Notification MMG v 2. 03 Tuberculosis Case Notification MMG v 1. 0 Varicella Case Notification MMG v 2. 0 Generic Case Notification MMG v 1. 0 Varicella Case Notification MMG v 1. 0 Arboviral Case Notification MMG v 1. 2 Generic Summary Case Notification MMG v 1. 0 # % 36 68. 6% 32 62. 7% 15 29. 4% 4 7. 8% # % 22 18 15 14 14 6 4 43. 1% 35. 3% 29. 4% 27. 5% 11. 8% 7. 8%

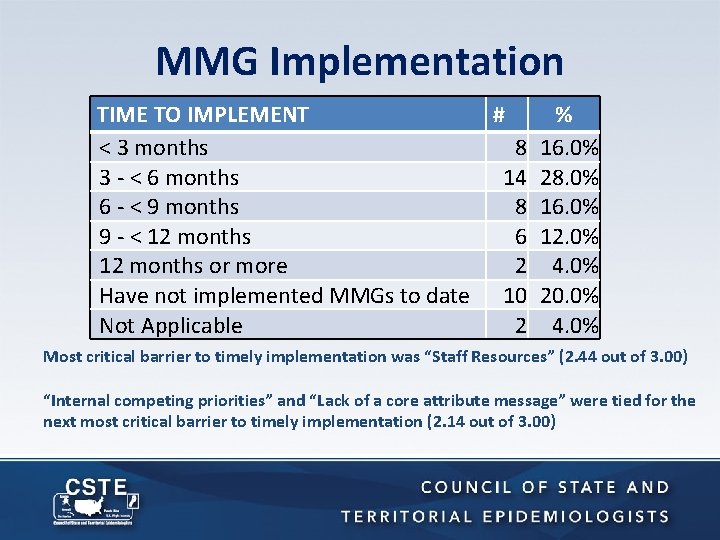
MMG Implementation TIME TO IMPLEMENT # % < 3 months 8 16. 0% 3 - < 6 months 14 28. 0% 6 - < 9 months 8 16. 0% 9 - < 12 months 6 12. 0% 12 months or more 2 4. 0% Have not implemented MMGs to date 10 20. 0% Not Applicable 2 4. 0% Most critical barrier to timely implementation was “Staff Resources” (2. 44 out of 3. 00) “Internal competing priorities” and “Lack of a core attribute message” were tied for the next most critical barrier to timely implementation (2. 14 out of 3. 00)

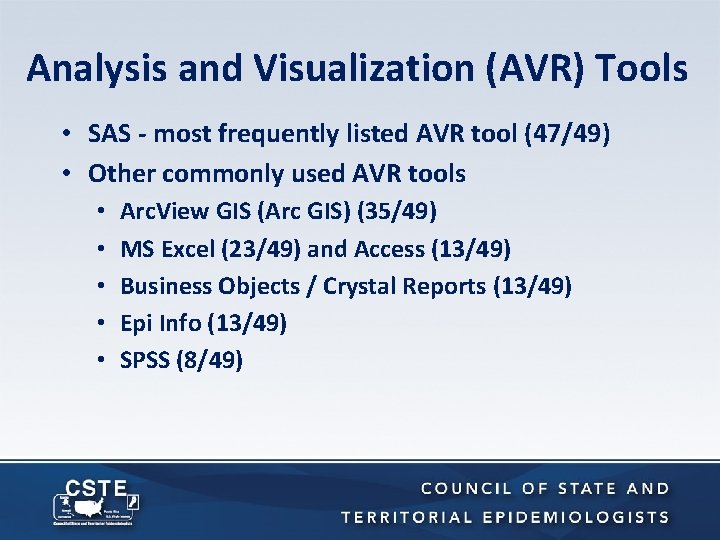
Analysis and Visualization (AVR) Tools • SAS - most frequently listed AVR tool (47/49) • Other commonly used AVR tools • • • Arc. View GIS (Arc GIS) (35/49) MS Excel (23/49) and Access (13/49) Business Objects / Crystal Reports (13/49) Epi Info (13/49) SPSS (8/49)

System Satisfaction SATISFACTION LEVEL Very satisfied Somewhat satisfied Neither satisfied nor dissatisfied Somewhat dissatisfied Very dissatisfied N=46 # % 21 45. 7% 22 47. 8% 1 2. 2% 2 4. 3% 0 0. 0%

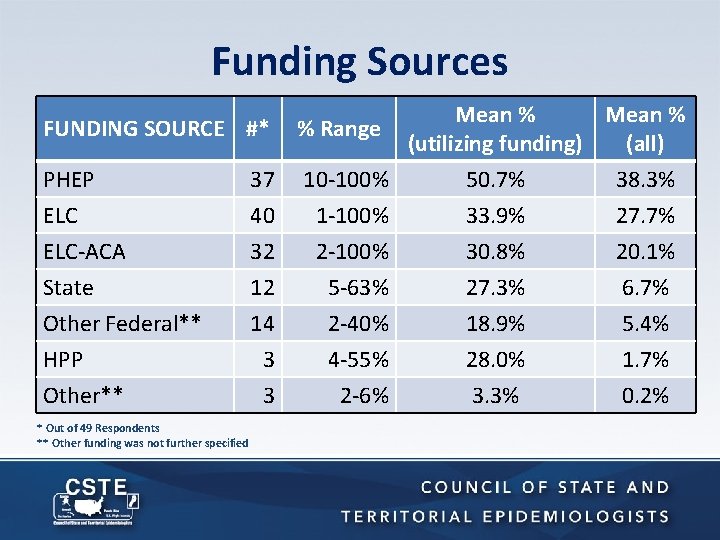
Funding Sources Mean % FUNDING SOURCE #* % Range (utilizing funding) (all) PHEP 37 10 -100% 50. 7% 38. 3% ELC 40 1 -100% 33. 9% 27. 7% ELC-ACA State Other Federal** HPP Other** * Out of 49 Respondents ** Other funding was not further specified 32 12 14 3 2 -100% 5 -63% 2 -40% 4 -55% 30. 8% 27. 3% 18. 9% 28. 0% 20. 1% 6. 7% 5. 4% 1. 7% 3 2 -6% 3. 3% 0. 2%

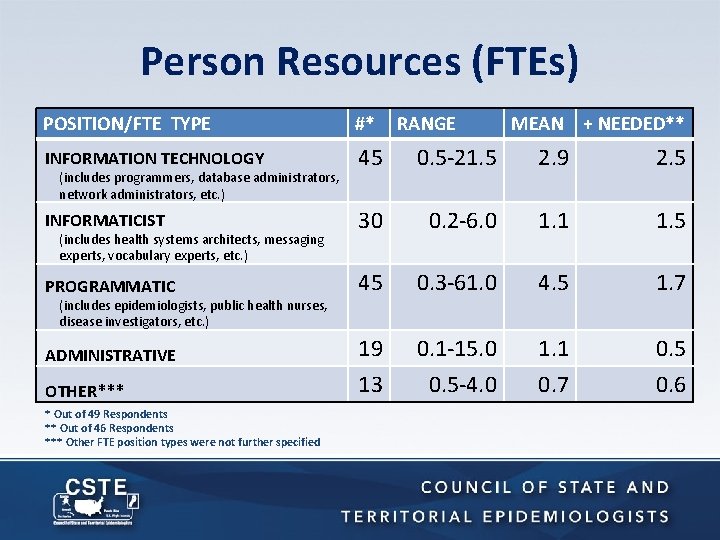
Person Resources (FTEs) POSITION/FTE TYPE #* INFORMATION TECHNOLOGY 45 0. 5 -21. 5 2. 9 2. 5 INFORMATICIST 30 0. 2 -6. 0 1. 1 1. 5 PROGRAMMATIC 45 0. 3 -61. 0 4. 5 1. 7 19 13 0. 1 -15. 0 0. 5 -4. 0 1. 1 0. 7 0. 5 0. 6 (includes programmers, database administrators, network administrators, etc. ) (includes health systems architects, messaging experts, vocabulary experts, etc. ) (includes epidemiologists, public health nurses, disease investigators, etc. ) ADMINISTRATIVE OTHER*** * Out of 49 Respondents ** Out of 46 Respondents *** Other FTE position types were not further specified RANGE MEAN + NEEDED**

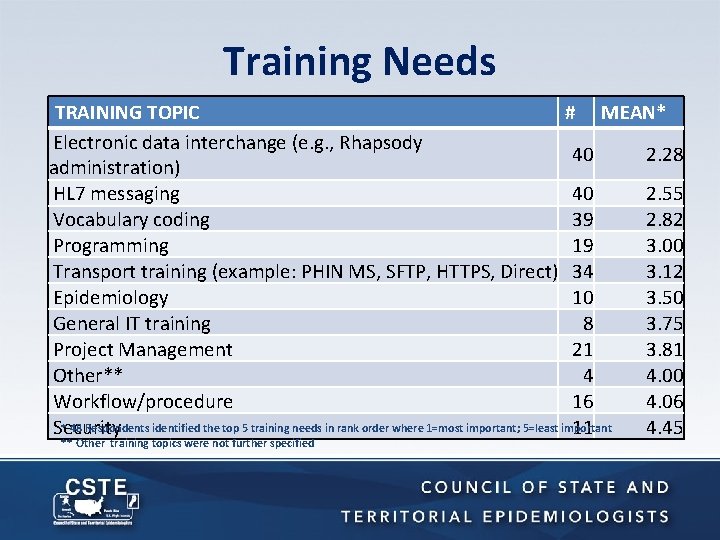
Training Needs TRAINING TOPIC # MEAN* Electronic data interchange (e. g. , Rhapsody 40 2. 28 administration) HL 7 messaging 40 2. 55 Vocabulary coding 39 2. 82 Programming 19 3. 00 Transport training (example: PHIN MS, SFTP, HTTPS, Direct) 34 3. 12 Epidemiology 10 3. 50 General IT training 8 3. 75 Project Management 21 3. 81 Other** 4 4. 00 Workflow/procedure 16 4. 06 * 48 Respondents identified the top 5 training needs in rank order where 1=most important; 5=least important Security 11 4. 45 ** Other training topics were not further specified

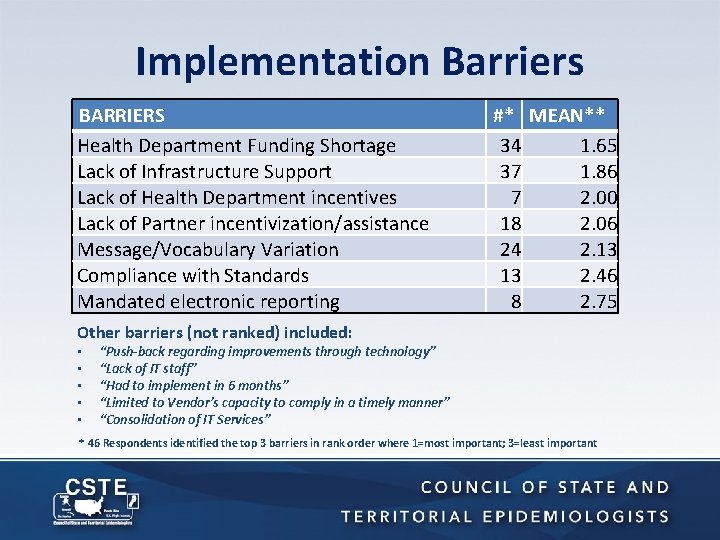
Implementation Barriers BARRIERS Health Department Funding Shortage Lack of Infrastructure Support Lack of Health Department incentives Lack of Partner incentivization/assistance Message/Vocabulary Variation Compliance with Standards Mandated electronic reporting #* MEAN** 34 1. 65 37 1. 86 7 2. 00 18 2. 06 24 2. 13 13 2. 46 8 2. 75 Other barriers (not ranked) included: • • • “Push-back regarding improvements through technology” “Lack of IT staff” “Had to implement in 6 months” “Limited to Vendor’s capacity to comply in a timely manner” “Consolidation of IT Services” * 46 Respondents identified the top 3 barriers in rank order where 1=most important; 3=least important

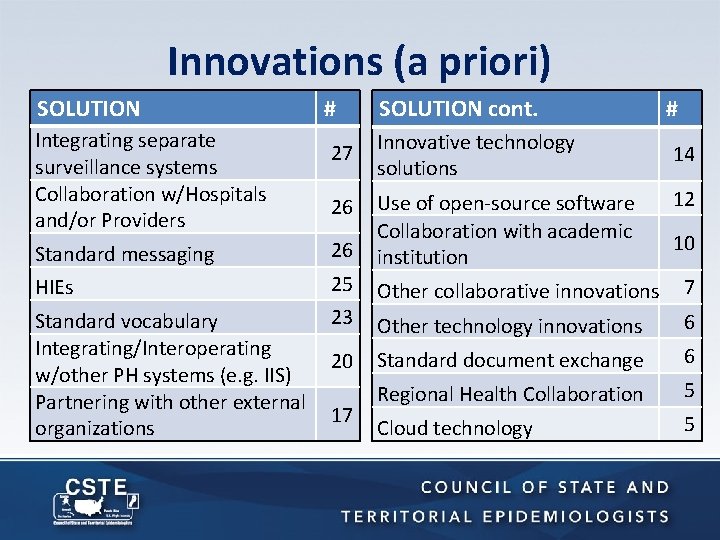
Innovations (a priori) SOLUTION Integrating separate surveillance systems Collaboration w/Hospitals and/or Providers # 27 26 Standard messaging 26 HIEs 25 Standard vocabulary Integrating/Interoperating w/other PH systems (e. g. IIS) Partnering with other external organizations 23 20 17 SOLUTION cont. # Innovative technology solutions 14 Use of open-source software Collaboration with academic institution 12 10 Other collaborative innovations 7 Other technology innovations 6 Standard document exchange 6 Regional Health Collaboration 5 Cloud technology 5
![Other Innovations State is utilizing the RCMT labs recorded in the NBS laboratory Other Innovations • “[State] is utilizing the RCMT, labs recorded in the NBS, laboratory](https://slidetodoc.com/presentation_image_h2/4f803e0d5a1fdd67a7aace8a992846f9/image-19.jpg)
Other Innovations • “[State] is utilizing the RCMT, labs recorded in the NBS, laboratory codes utilized by LTIAPH and partners, and CDC case definitions to create a [state] preferred list of SNOMED and LOINC” • “The [state] patient portal should help to supplement limited investigative resources (FTE). ” • “[State] established a division to integrate surveillance across all infectious disease and support informatics initiatives for the bureau of infectious disease. This allows unified and single approach to surveillance, working with local health, electronic laboratory reporting and HIE. ”

Conclusions • Increase in NEDSS-compatible systems in production • 80% in 2007 to 98% in 2012 • Increase in systems able to receive ELR • 70% in 2007 to 96% in 2012 • Increase in systems able to receive public health case report • 54% in 2007 to 63% in 2012 • Funding remains the main barrier to electronic surveillance system implementation • Electronic data exchange training needed

Acknowledgements • CSTE NEDSS Assessment Workgroup: Rita Altamore (WA), June Bancroft (OR), Susan Bohm (MI), Meredith Bradbury (CO), Jim Collins (MI), Todd Davis (IL), Ed Hartwick (MI), Lesliann Helmus (VA), Shannon Johnson (MI), Anthony Lee (OK), Tracy Miller (ND), Sandy Roush (CDC), Marjorie Shannon (DE), Del Williams (NC) ROUNDTABLE ON WEDNESDAY 1 -1: 45 RM 212
 Annie fine cste
Annie fine cste Perform surveillance without electronic devices
Perform surveillance without electronic devices Regulatory agencies
Regulatory agencies Communicable disease and non communicable disease
Communicable disease and non communicable disease An electronic is the electronic exchange of money or scrip
An electronic is the electronic exchange of money or scrip Electronic news gathering and electronic field production
Electronic news gathering and electronic field production Texas behavioral risk factor surveillance system
Texas behavioral risk factor surveillance system Continuing analysis and surveillance system
Continuing analysis and surveillance system Pss pro surveillance system
Pss pro surveillance system Video surveillance system
Video surveillance system Video surveillance system
Video surveillance system National patient safety goals 2012
National patient safety goals 2012 National research university of electronic technology
National research university of electronic technology Renns autentificare
Renns autentificare Who rsv surveillance
Who rsv surveillance Surveillance post opératoire
Surveillance post opératoire Types of surveillance
Types of surveillance Surveillance fixateur externe
Surveillance fixateur externe Reconnaissance and surveillance leaders course
Reconnaissance and surveillance leaders course Primary surveillance radar (psr)
Primary surveillance radar (psr) Sentinel surveillance definition
Sentinel surveillance definition Drain pedinielli
Drain pedinielli









































