Critical Material Properties for Pharmaceutical Dosage Forms Industry


- Slides: 20

Critical Material Properties for Pharmaceutical Dosage Forms - Industry Perspective Tony Hlinak Abbott Laboratories North Chicago, IL

Real World – Case 1 Spec Limits Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott ? 2

Real World – Case 2 ? Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 3

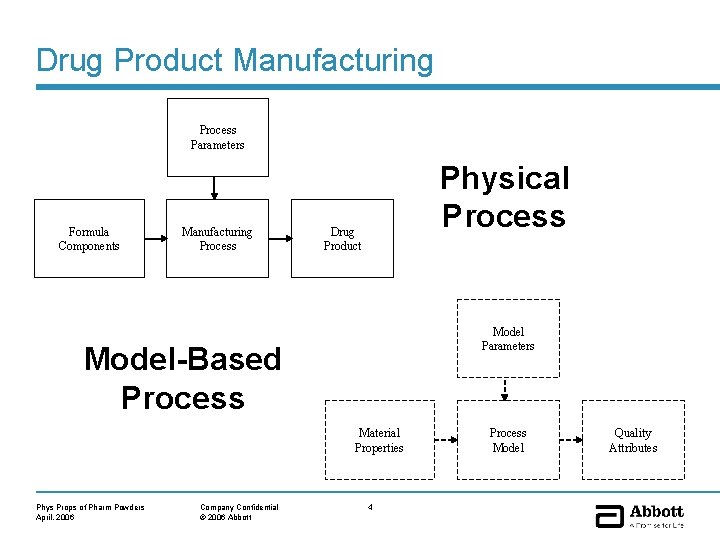
Drug Product Manufacturing Process Parameters Formula Components Manufacturing Process Physical Process Drug Product Model Parameters Model-Based Process Material Properties Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 4 Process Model Quality Attributes

Material Properties Require Attention • Affect processing behavior and final dose performance • Can compromise an otherwise robust process – Reduce Reliability – Make Less Predictable • Minimum Costs – Excessive waste, chronic rework, increased cycle times, uneven utilization of resources, and increased compliance risk • Worst Case – Disrupt supply chain, exhaust technical and management resources, threaten relationship with regulatory agencies, weaken competitive position, and ultimately lose customers Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 5

Material Property Effects - Realities • Complex, Broad, Interrelated • Many Characteristics Not True “Properties” in Thermodynamic Sense • Relevant Property Information Not Available for Many Materials Even Those That are Widely Used in our Industry • Many Properties of Interest Not Measurable Using Conventional Analytical Tools • Variability in Some Methods are High and/or Highly Technique Dependent • Property Impacts May Change with Processing Scale • Property Impacts Depend on Both Amount Present in Formula and Other Components Present Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 6

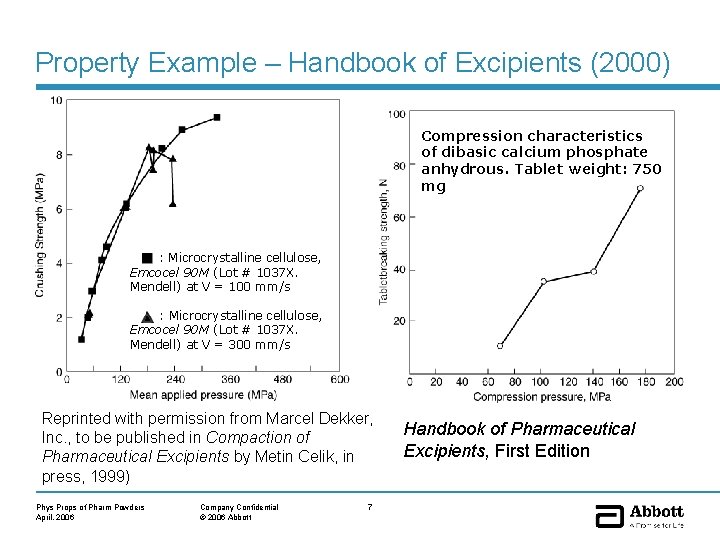
Property Example – Handbook of Excipients (2000) Compression characteristics of dibasic calcium phosphate anhydrous. Tablet weight: 750 mg : Microcrystalline cellulose, Emcocel 90 M (Lot # 1037 X. Mendell) at V = 100 mm/s : Microcrystalline cellulose, Emcocel 90 M (Lot # 1037 X. Mendell) at V = 300 mm/s Reprinted with permission from Marcel Dekker, Inc. , to be published in Compaction of Pharmaceutical Excipients by Metin Celik, in press, 1999) Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 7 Handbook of Pharmaceutical Excipients, First Edition

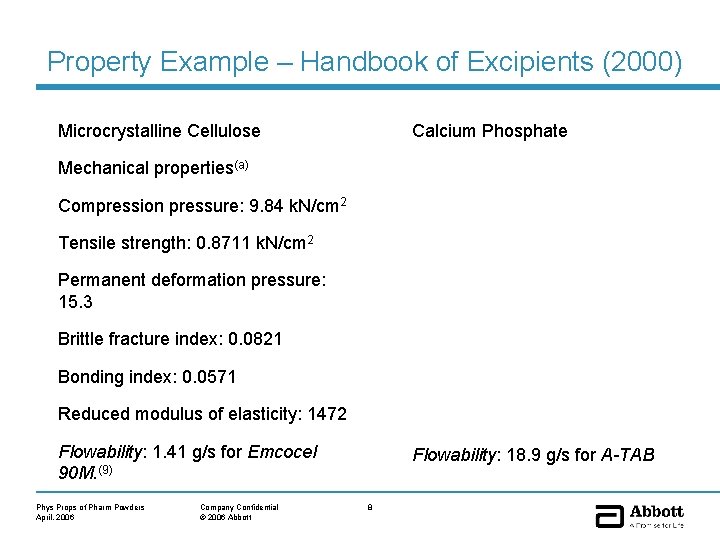
Property Example – Handbook of Excipients (2000) Microcrystalline Cellulose Calcium Phosphate Mechanical properties(a) Compression pressure: 9. 84 k. N/cm 2 Tensile strength: 0. 8711 k. N/cm 2 Permanent deformation pressure: 15. 3 Brittle fracture index: 0. 0821 Bonding index: 0. 0571 Reduced modulus of elasticity: 1472 Flowability: 1. 41 g/s for Emcocel 90 M. (9) Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott Flowability: 18. 9 g/s for A-TAB 8

The Current State 1) Little or no information concerning relevant properties may be available on a particular component even those that are widely used within the industry 2) The properties of interest may not be measurable using conventional analytical tools or the results obtained are very technique dependent 3) Reliable mixing rules that allow estimates of mixture properties to be generated from the known properties of the components don’t generally exist 4) Physical models that link the output properties to the input properties are currently insufficient for most pharmaceutical unit operations 5) The impact of a particular property may change as the pharmaceutical manufacturing process is scaled or optimized, and will likely depend on both the amount of the component present in the formula as well as the type and amount of other components present Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 9

The Flow Property Group Particle Size Distribution Particle Shape Distribution Bulk Density Surface Area Surface Energy Cohesiveness Surface Structure Static Charge Hygroscopicity Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 10

The Uniformity Property Group Particle Size Distribution Particle Shape Distribution Surface Area Surface Energy Cohesiveness Surface Structure Static Charge Hygroscopicity Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 11

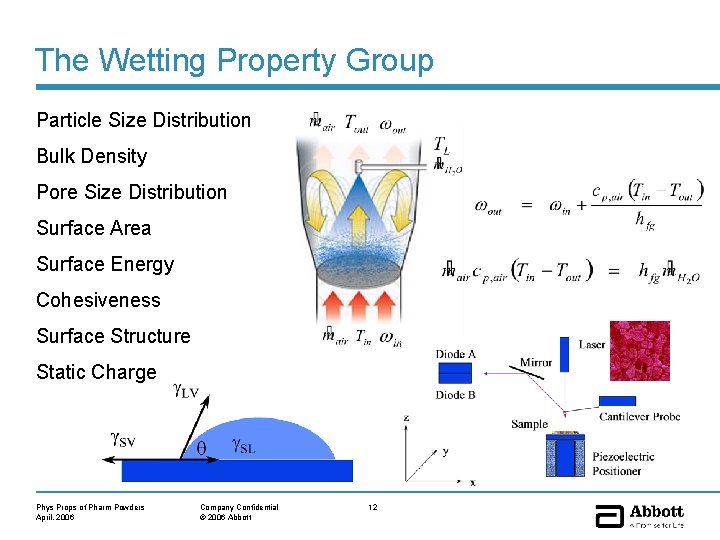
The Wetting Property Group Particle Size Distribution Bulk Density Pore Size Distribution Surface Area Surface Energy Cohesiveness Surface Structure Static Charge Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 12

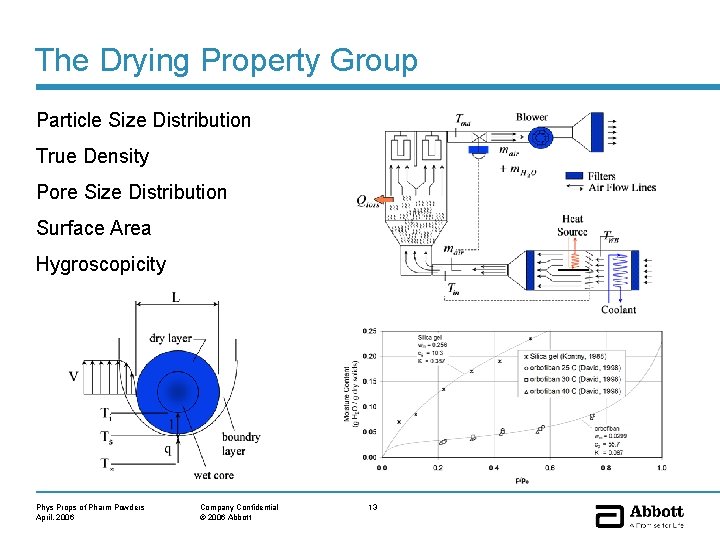
The Drying Property Group Particle Size Distribution True Density Pore Size Distribution Surface Area Hygroscopicity Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 13

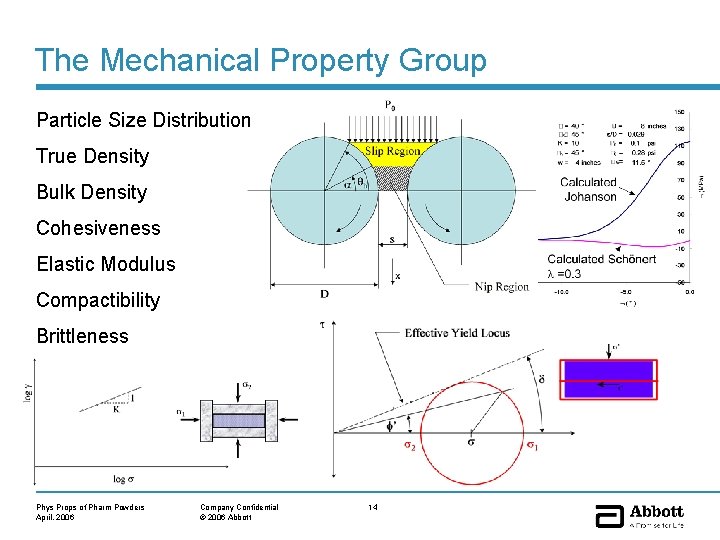
The Mechanical Property Group Particle Size Distribution True Density Bulk Density Cohesiveness Elastic Modulus Compactibility Brittleness Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 14

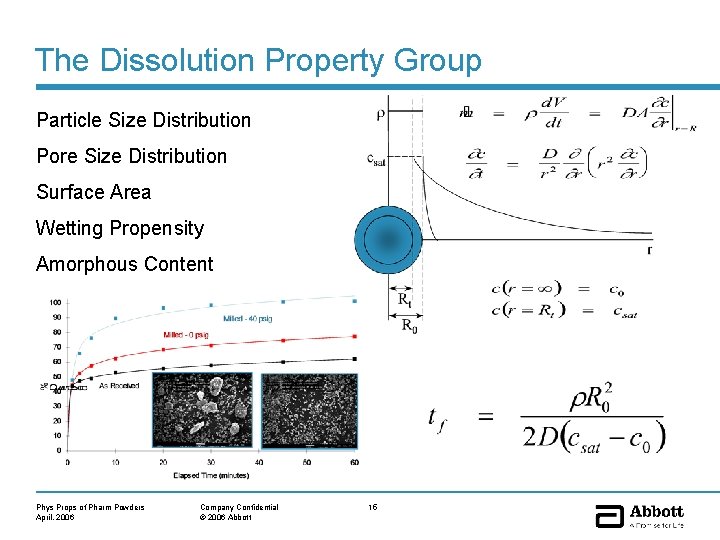
The Dissolution Property Group Particle Size Distribution Pore Size Distribution Surface Area Wetting Propensity Amorphous Content Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 15

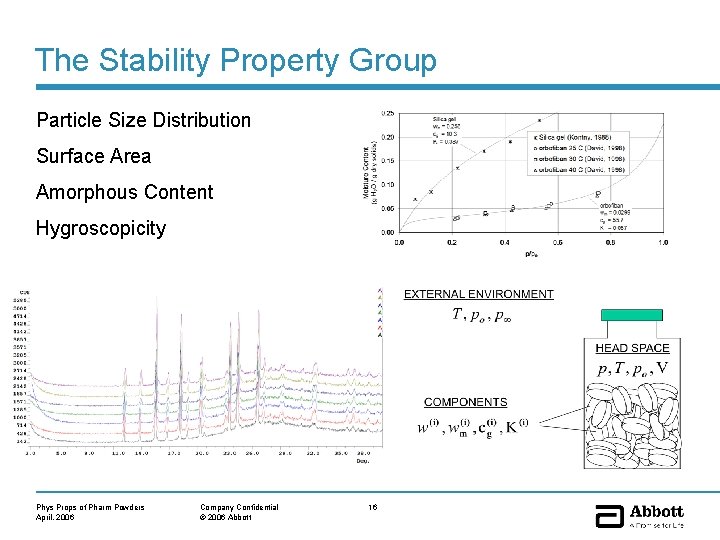
The Stability Property Group Particle Size Distribution Surface Area Amorphous Content Hygroscopicity Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 16

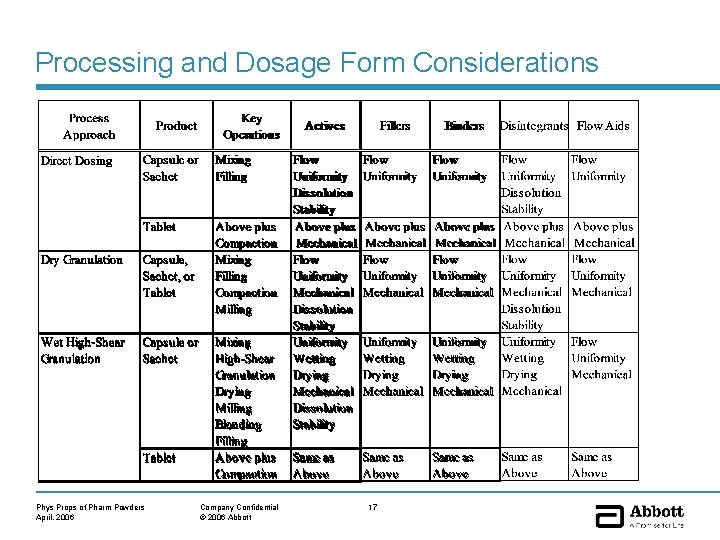
Processing and Dosage Form Considerations Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 17

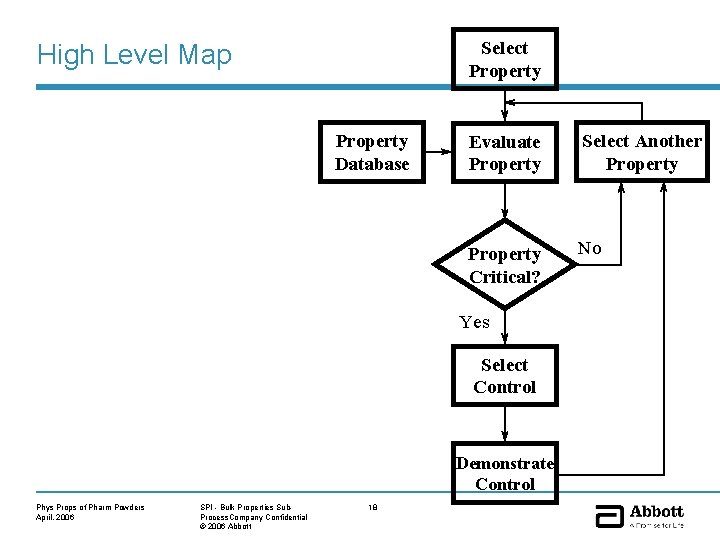
Select Property High Level Map Particle Size Property Database Cohesiveness Bulk Density Surface Structure Surface Area Amorphous Content Particle Shape Tensile Strength True Density Elastic Moduli Pore Size Compactibility Surface Energy Brittleness Flow Property Static Charge Select Another Property Critical? No Yes Select Control Wetting Phys Props of Pharm Powders April, 2006 Evaluate Property Demonstrate Control SPI - Bulk Properties Sub. Process. Company Confidential © 2006 Abbott 18

Recommendations 1. Currently available models for common pharmaceutical unit operations should be assessed and the relevant physical properties extracted 2. Available methods for quantifying the physical property list generated in the step above should be evaluated and the most promising approaches further developed specifically for pharmaceutical powders 3. As methods reach a sufficient state of maturity, the most commonly used pharmaceutical materials should be characterized and the results published in standardized tables. The results should include multiple vendors and include appropriate estimates of variation 4. The component results and the methods from the step above should be used to generate appropriate mixing rules for predicting the relevant properties of powder mixtures from the properties of the components 5. Iterate Phys Props of Pharm Powders April, 2006 Company Confidential © 2006 Abbott 19
