Conversions and Significant Figures Kelley Kuhn Center for






- Slides: 11

Conversions and Significant Figures Kelley Kuhn Center for Creative Arts

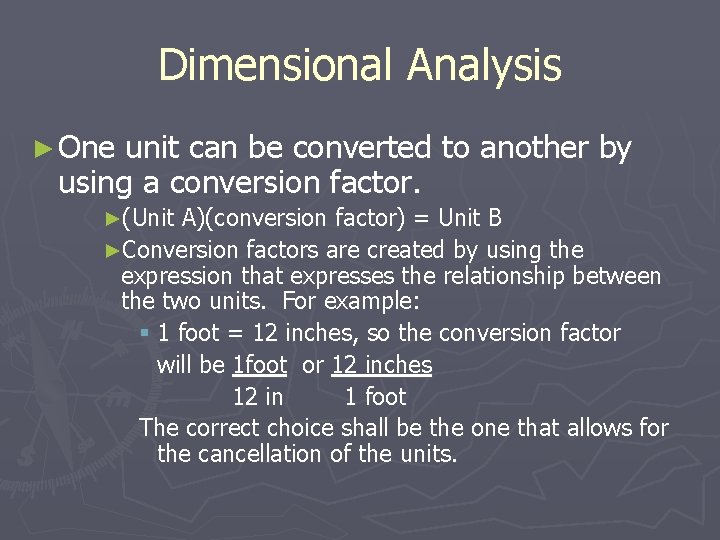
Dimensional Analysis ► One unit can be converted to another by using a conversion factor. ►(Unit A)(conversion factor) = Unit B ►Conversion factors are created by using the expression that expresses the relationship between the two units. For example: § 1 foot = 12 inches, so the conversion factor will be 1 foot or 12 inches 12 in 1 foot The correct choice shall be the one that allows for the cancellation of the units.

Practice Conversions ► Convert the following quantities from one unit to another using the following relationships. ► 1 m = 1. 094 yd 1 mile = 1760 yd ► 30 m to mil ► 1500 yd to mil ► 206 mil to m ► 34 kg to lbs ► 34 lb to kg 1 kg = 2. 205 lbs

Density is an important physical characteristic in Chemistry. It is often used to aid in the identification of unknown solids and liquids. Density = mass/volume Because liquids are frequently used in the lab setting, a volume measured in graduated cylinder can be used to determine the mass using density. Mass = (density)(volume)

“Certainty” in Measurement ► When visually reading the measurement using a lab tool such as a graduated cylinder or meter stick, there is always a certain degree of uncertainty in the recorded quantity. The reading may fall between two divisions on the scale and an estimate must be made in order to record the final digit.

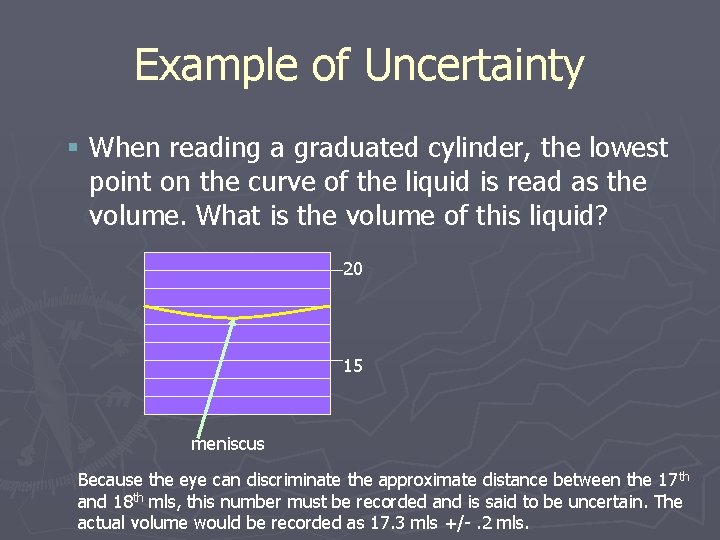
Example of Uncertainty § When reading a graduated cylinder, the lowest point on the curve of the liquid is read as the volume. What is the volume of this liquid? 20 15 meniscus Because the eye can discriminate the approximate distance between the 17 th and 18 th mls, this number must be recorded and is said to be uncertain. The actual volume would be recorded as 17. 3 mls +/-. 2 mls.

Are Significant Figures Important? A Fable ► ► ► A student once needed a cube of metal which had to have a mass of 83 grams. He knew the density of this metal was 8. 67 g/m. L, which told him the cube's volume. Believing significant figures were invented just to make life difficult for chemistry students and had no practical use in the real world, he calculated the volume of the cube as 9. 573 m. L. He thus determined that the edge of the cube had to be 2. 097 cm. He took his plans to the machine shop where his friend had the same type of work done the previous year. The shop foreman said, "Yes, we can make this according to your specifications - but it will be expensive. " "That's OK, " replied the student. "It's important. " He knew his friend has paid $35, and he had been given $50 out of the school's research budget to get the job done. He returned the next day, expecting the job to be done. "Sorry, " said the foreman. "We're still working on it. Try next week. " Finally the day came, and our friend got his cube. It looked very, very smooth and shiny and beautiful in its velvet case. Seeing it, our hero had a premonition of disaster and became a bit nervous. But he summoned up enough courage to ask for the bill. "$500, and cheap at the price. We had a terrific job getting it right -- had to make three before we got one right. " "But--but--my friend paid only $35 for the same thing!" "No. He wanted a cube 2. 1 cm on an edge, and your specifications called for 2. 097. We had yours roughed out to 2. 1 that very afternoon, but it was the precision grinding and lapping to get it down to 2. 097 which took so long and cost the big money. The first one we made was 2. 089 on one edge when we got finshed, so we had to scrap it. The second was closer, but still not what you specified. That's why the three tries. " "Oh!"

Significant Figures ► The certain and uncertain numbers that must be recorded are called significant figures. ► Significant figures (sigfigs) are important for accurately conveying experimental data. ► Sigfig rules help to identify which numbers have meaning and which are simply placeholders.

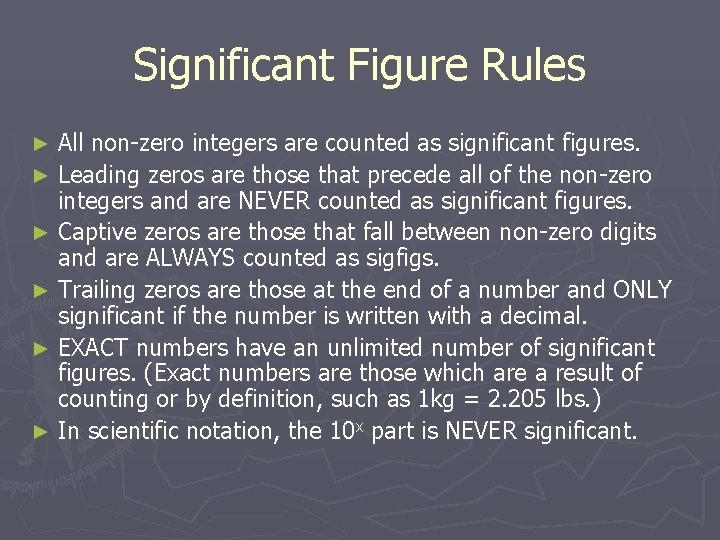
Significant Figure Rules All non-zero integers are counted as significant figures. ► Leading zeros are those that precede all of the non-zero integers and are NEVER counted as significant figures. ► Captive zeros are those that fall between non-zero digits and are ALWAYS counted as sigfigs. ► Trailing zeros are those at the end of a number and ONLY significant if the number is written with a decimal. ► EXACT numbers have an unlimited number of significant figures. (Exact numbers are those which are a result of counting or by definition, such as 1 kg = 2. 205 lbs. ) ► In scientific notation, the 10 x part is NEVER significant. ►

Using Sigfigs in Calculations ► When multiplying or dividing, limit the answer to the same number of significant figures that appear in the original data with the fewest number of sigfigs. ► When adding or subtracting, limit the answer to the same number of decimal places that appear in the original data with the fewest number of decimal places.

Let’s Try It! ► How many sigfigs in these numbers? § 0. 00970, 130400, 0. 203040, 7. 650 x 10 -8 § 3, 4, 6, 4 Record the answer to the following in correct sigfigs. 10. 045 +. 33. 00976 – 0. 0100 (34. 00)(0. 001) 76. 2/0. 1000
 Thomas kuhn elizabeth kuhn
Thomas kuhn elizabeth kuhn Upper and lower bounds to 3 significant figures
Upper and lower bounds to 3 significant figures Upper and lower bounds
Upper and lower bounds Accuracy, precision, and significant figures worksheet
Accuracy, precision, and significant figures worksheet Upper and lower bounds of rounded numbers
Upper and lower bounds of rounded numbers Errors and significant figures
Errors and significant figures Congruent figures and similar figures
Congruent figures and similar figures Flat shape with eight sides
Flat shape with eight sides Washing machine solid or plane figure
Washing machine solid or plane figure Significant figures ap chemistry
Significant figures ap chemistry Rules for significant figures
Rules for significant figures Significant
Significant