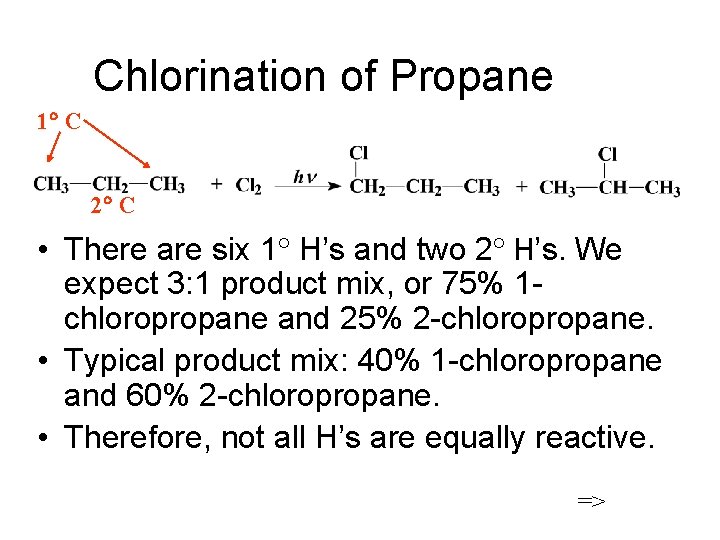
Chlorination of Propane 1 C 2 C There
- Slides: 10

Chlorination of Propane 1 C 2 C • There are six 1 H’s and two 2 H’s. We expect 3: 1 product mix, or 75% 1 chloropropane and 25% 2 -chloropropane. • Typical product mix: 40% 1 -chloropropane and 60% 2 -chloropropane. • Therefore, not all H’s are equally reactive. =>

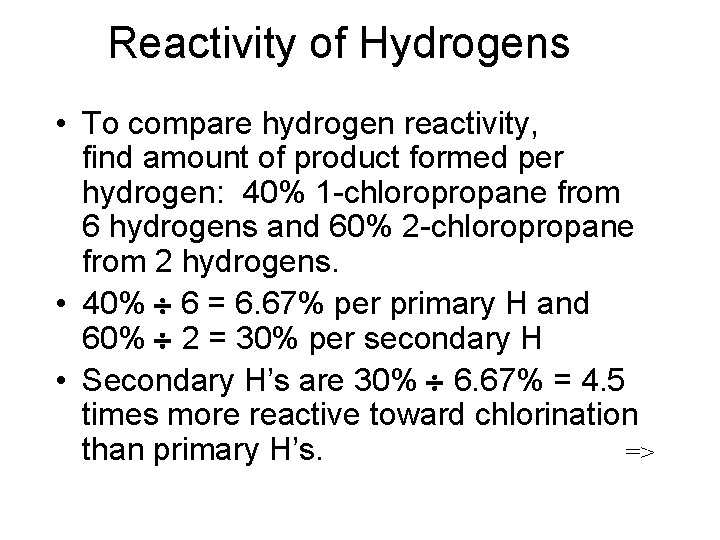
Reactivity of Hydrogens • To compare hydrogen reactivity, find amount of product formed per hydrogen: 40% 1 -chloropropane from 6 hydrogens and 60% 2 -chloropropane from 2 hydrogens. • 40% 6 = 6. 67% per primary H and 60% 2 = 30% per secondary H • Secondary H’s are 30% 6. 67% = 4. 5 times more reactive toward chlorination than primary H’s. =>

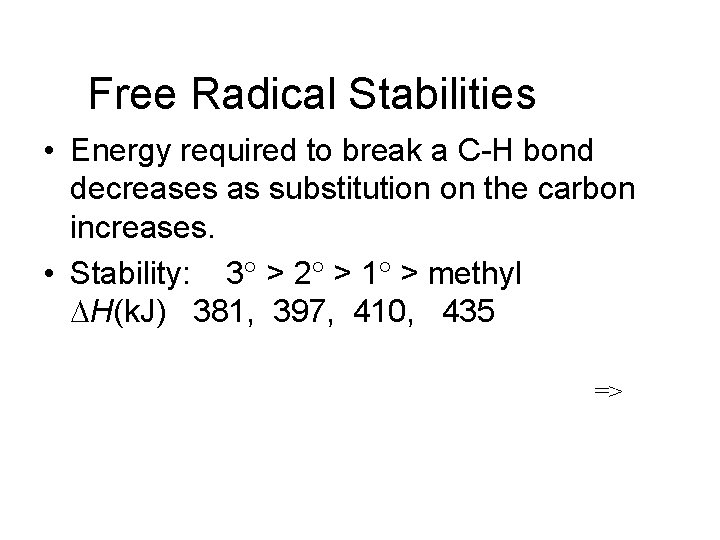
Free Radical Stabilities • Energy required to break a C-H bond decreases as substitution on the carbon increases. • Stability: 3 > 2 > 1 > methyl DH(k. J) 381, 397, 410, 435 =>

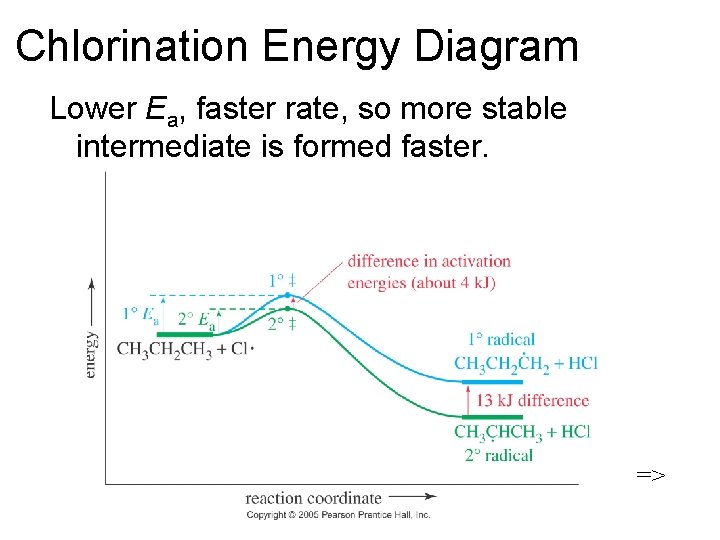
Chlorination Energy Diagram Lower Ea, faster rate, so more stable intermediate is formed faster. =>

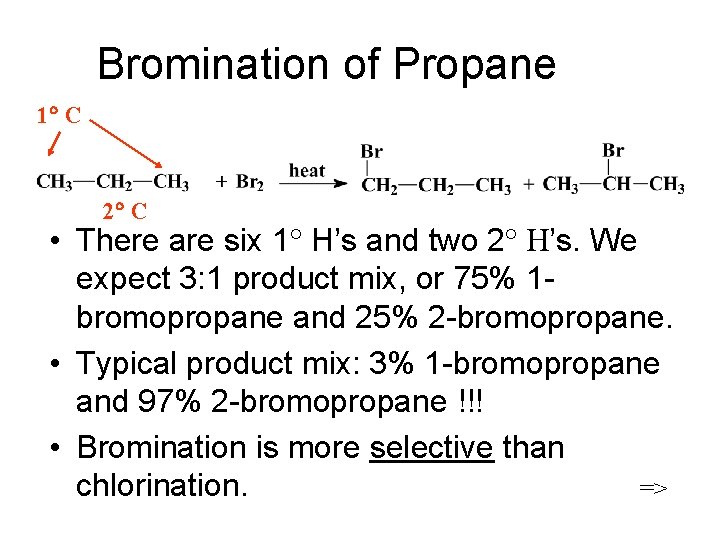
Bromination of Propane 1 C 2 C • There are six 1 H’s and two 2 H’s. We expect 3: 1 product mix, or 75% 1 bromopropane and 25% 2 -bromopropane. • Typical product mix: 3% 1 -bromopropane and 97% 2 -bromopropane !!! • Bromination is more selective than chlorination. =>

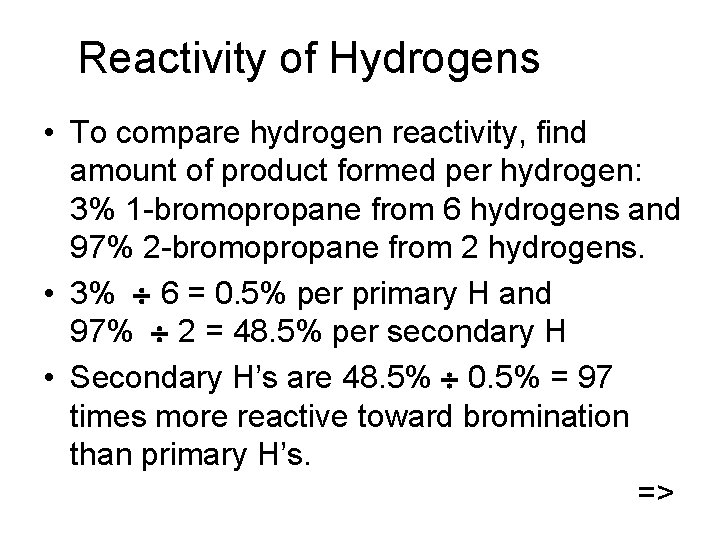
Reactivity of Hydrogens • To compare hydrogen reactivity, find amount of product formed per hydrogen: 3% 1 -bromopropane from 6 hydrogens and 97% 2 -bromopropane from 2 hydrogens. • 3% 6 = 0. 5% per primary H and 97% 2 = 48. 5% per secondary H • Secondary H’s are 48. 5% 0. 5% = 97 times more reactive toward bromination than primary H’s. =>

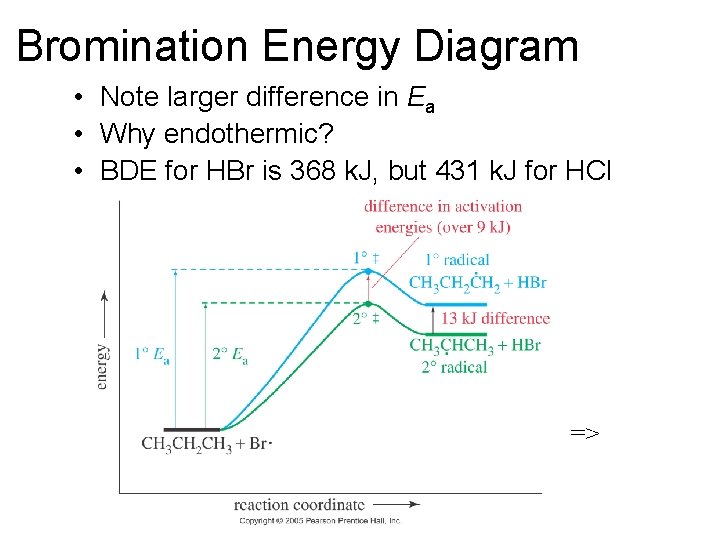
Bromination Energy Diagram • Note larger difference in Ea • Why endothermic? • BDE for HBr is 368 k. J, but 431 k. J for HCl =>

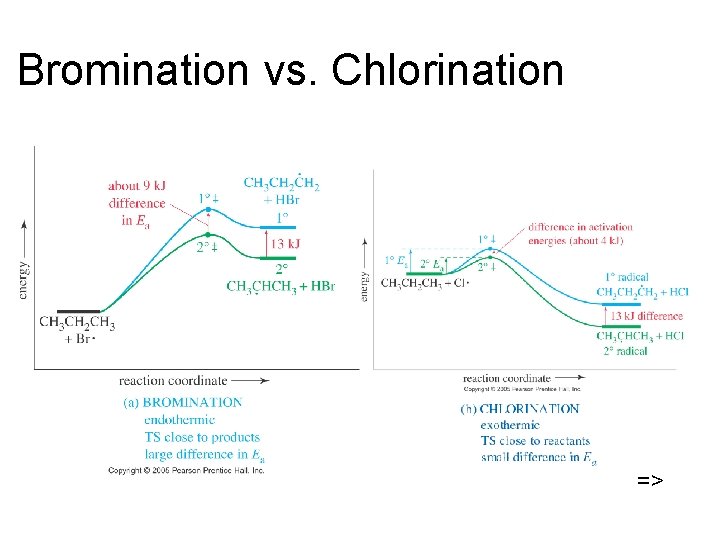
Bromination vs. Chlorination =>

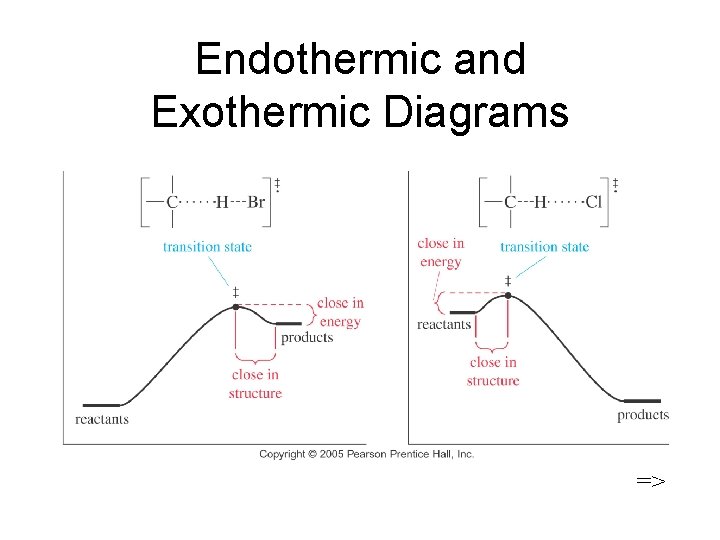
Endothermic and Exothermic Diagrams =>

Hammond Postulate • Related species that are similar in energy are also similar in structure. The structure of a transition state resembles the structure of the closest stable species. • Endothermic reaction: transition state is product-like. • Exothermic reaction: transition state is reactant-like. =>
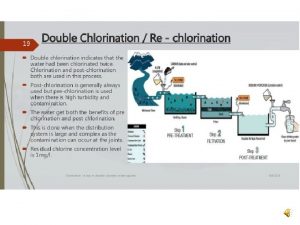
 Objective of disinfection
Objective of disinfection Purification of water on large scale storage
Purification of water on large scale storage Xxchx
Xxchx Lucas test reagent
Lucas test reagent Strong electrophile examples
Strong electrophile examples Breakpoint chlorination definition
Breakpoint chlorination definition Difference between slow and rapid sand filter
Difference between slow and rapid sand filter Propane injection for gas engines
Propane injection for gas engines The weekly demand for propane gas
The weekly demand for propane gas Propane heat exchanger
Propane heat exchanger Routing software for propane
Routing software for propane