Chemistry Chapter 2 Analyzing Data Metric System Systme









- Slides: 15

Chemistry Chapter 2: Analyzing Data

Metric System • Système Internationale d'Unités (SI) is an internationally agreed upon system of measurements (aka the metric system). • People all over the world can communicate about measurements regardless of country, language, etc. • A base unit is a defined unit in a system of measurement that is based on an object or event in the physical world, and is independent of other units.

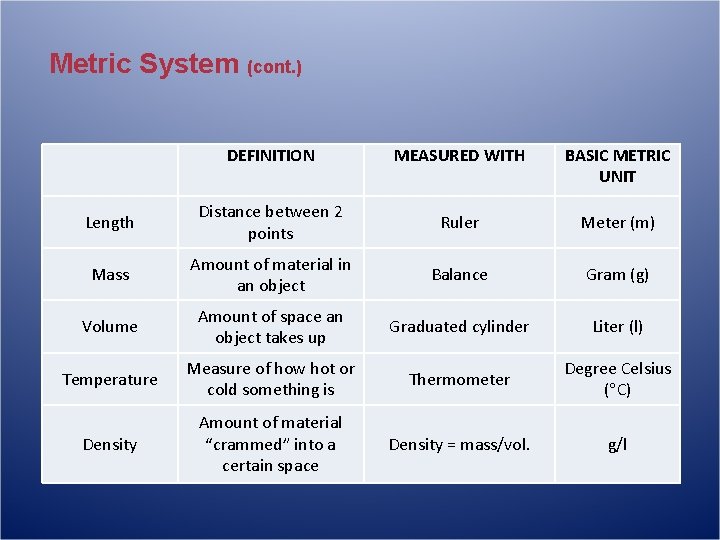
Metric System (cont. ) DEFINITION MEASURED WITH BASIC METRIC UNIT Length Distance between 2 points Ruler Meter (m) Mass Amount of material in an object Balance Gram (g) Volume Amount of space an object takes up Graduated cylinder Liter (l) Temperature Measure of how hot or cold something is Thermometer Degree Celsius ( C) Density Amount of material “crammed” into a certain space Density = mass/vol. g/l

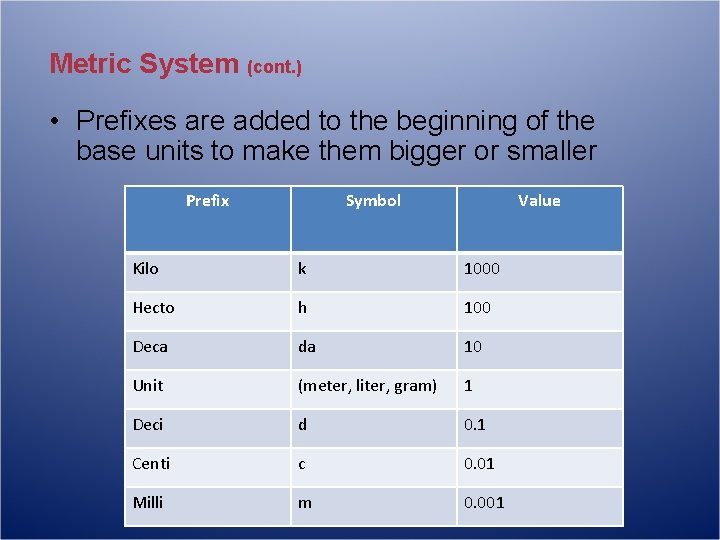
Metric System (cont. ) • Prefixes are added to the beginning of the base units to make them bigger or smaller Prefix Symbol Value Kilo k 1000 Hecto h 100 Deca da 10 Unit (meter, liter, gram) 1 Deci d 0. 1 Centi c 0. 01 Milli m 0. 001

Derived Units • Not all quantities can be measured with SI base units or a piece of lab equipment. • A unit that is defined by a combination of base units is called a derived unit. • Density is a derived unit, g/cm 3, the amount of mass per unit volume. • The density equation is density = mass/volume

Converting Between Metric Units • Use the following figure to help you hop from one metric unit to another • Move the decimal point the same number of times and in the same direction you hopped King Henry Doesn’t Usually Drink Chocolate Milk Kilo - Hecto - Deca - Unit – Deci – Centi - Milli

Converting Between Metric Units (cont. ) k - h - da - unit - d - c - m Kilo - Hecto - Deca - Unit – Deci – Centi – Milli meter, liter, gram Examples: 25. 3 kg = 25, 300 g 1. 26 m = 1260 mm 1500 ml = 0. 01500 hl 100 cg = 0. 00100 kg

Scientific Notation • Scientific notation can be used to express any number as a number between 1 and 10 (the coefficient) multiplied by 10 raised to a power (the exponent). • Count the number of places the decimal point must be moved to give a coefficient between 1 and 10.

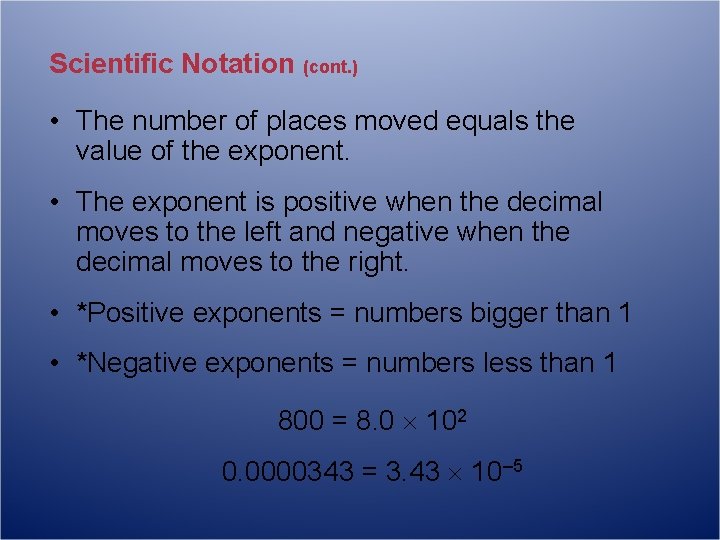
Scientific Notation (cont. ) • The number of places moved equals the value of the exponent. • The exponent is positive when the decimal moves to the left and negative when the decimal moves to the right. • *Positive exponents = numbers bigger than 1 • *Negative exponents = numbers less than 1 800 = 8. 0 102 0. 0000343 = 3. 43 10– 5

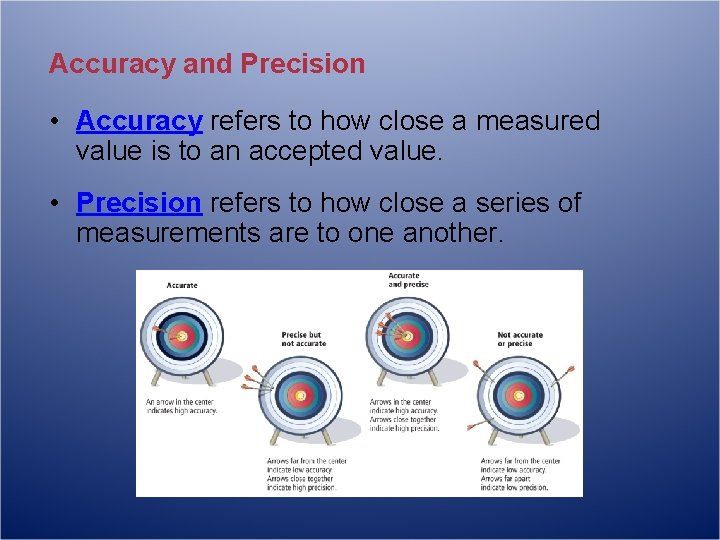
Accuracy and Precision • Accuracy refers to how close a measured value is to an accepted value. • Precision refers to how close a series of measurements are to one another.

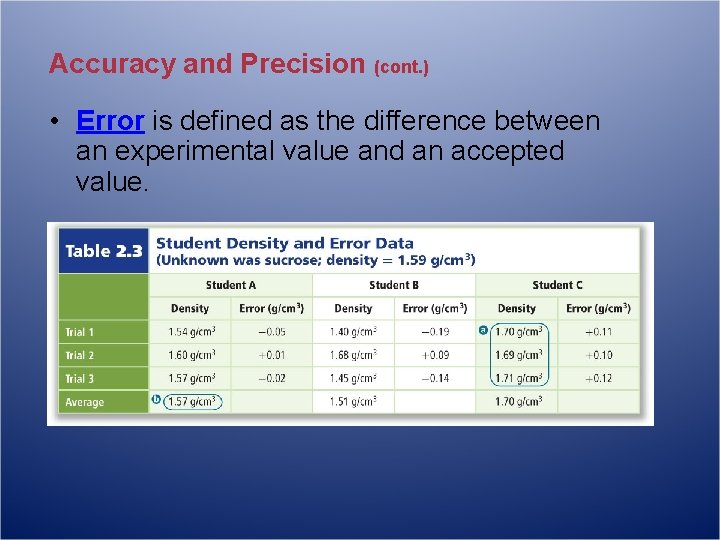
Accuracy and Precision (cont. ) • Error is defined as the difference between an experimental value and an accepted value.

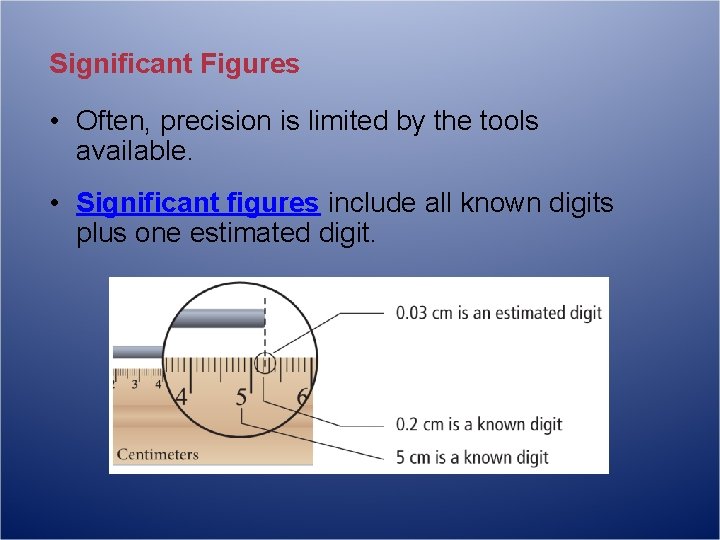
Significant Figures • Often, precision is limited by the tools available. • Significant figures include all known digits plus one estimated digit.

Significant Figures (cont. ) • Rules for significant figures – Rule 1: Nonzero numbers are always significant. – Rule 2: Zeros between nonzero numbers are always significant. – Rule 3: All final zeros to the right of the decimal are significant. – Rule 4: Zeros at the beginning of a number are not significant. – Rule 5: Counting numbers and defined constants have an infinite number of significant figures.

Rounding Numbers • Calculators are not aware of significant figures. • Answers should not have more significant figures than the original data with the fewest figures, and should be rounded.

Rounding Numbers (cont. ) • Addition and subtraction – Round numbers so all numbers have the same number of digits to the right of the decimal. • Multiplication and division – Round the answer to the same number of significant figures as the original measurement with the fewest significant figures.