Chemical Thermodynamics 20182019 4 th Lecture Manipulations of














- Slides: 14

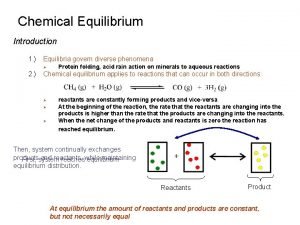
Chemical Thermodynamics 2018/2019 4 th Lecture: Manipulations of the 1 st Law and Adiabatic Changes Valentim M B Nunes, UD de Engenharia

Relations between partial derivatives Partial derivatives have many useful properties, and we can use it to manipulate the functions related with the first Law to obtain very useful thermodynamic relations. Let us recall some of those properties. If f is a function of x and y, f = f(x, y), then If z is a variable on which x and y depend, then 2

Relations between partial derivatives The Inverter: The Permuter: Euler’s chain relation: Finally, the differential df = g dx + h dy is exact, if: 3

Changes in Internal Energy Recall that U = U(T, V). So when T and V change infinitesimally The partial derivatives have already a physical meaning (remember last lecture), so: 4

Change of U with T at constant pressure What does this mean? Using the relation of slide 2 we can writhe: We define the isobaric thermal expansion coefficient as Finally we obtain: = 0 for an ideal gas Proofs relation between Cp and Cv for an ideal gas! Closed system at constant pressure and fixed composition! 5

Change of H with T at constant volume Let us choose H = H(T, p). This implies that Now we will divide everything by d. T, and impose constant volume What is the meaning of this two partial derivatives? 6

Change of H with T at constant volume Using the Euler’s relation Rearranging What does this mean? We define now the isothermal compressibility coefficient To assure that k. T is positive! So, we find that 7

Change of H with T at constant volume Using again the Euler’s relation and rearranging or We finally obtain What is this? See next slide! For now we will call it µJT 8

The Joule-Thomson Expansion Consider the fast expansion of a gas trough a throttle: If Q = 0 (adiabatic) then So, by the definition of enthalpy Isenthalpic process! 9

The Joule-Thomson effect For an ideal gas, µJT = 0. For most real gases Tinv >> 300 K. If µJT >0 the gas cools upon expansion (refrigerators). If µJT <0 then the gas heats up upon expansion. 10

Adiabatic expansion of a perfect gas From the 1 st Law, d. U = dq + dw. For an adiabatic process d. U = dw and d. U = Cvd. T, so for any expansion (or compression): For an irreversible process, against constant pressure: The gas cools! 11

Adiabatic expansion of a perfect gas For a reversible process, CVd. T = -pd. V along the path. Now, per mole, for an ideal gas, PV = RT, so For an ideal gas, Cp-Cv = R, and introducing then The gas cools! 12

Adiabatic expansion of a perfect gas We can now obtain an equivalent equation in terms of the pressure: As a conclusion, is constant along a reversible adiabatic. For instance, for a monoatomic ideal gas, 13

Adiabatic vs isothermal expansion 14
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Thermodynamics chemical equilibrium
Thermodynamics chemical equilibrium 熱力
熱力 Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Chemical engineering thermodynamics 8th solution chapter 2
Chemical engineering thermodynamics 8th solution chapter 2 Chemical engineering thermodynamics 8th solution chapter 4
Chemical engineering thermodynamics 8th solution chapter 4 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Modern chemistry chapter 7 review answers
Modern chemistry chapter 7 review answers Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Are kc and kp equal
Are kc and kp equal