Chapter 8 CIC and Chapter 4 20 CTCS






- Slides: 14

Chapter 8 (CIC) and Chapter 4, 20 (CTCS) • Read in CTCS Chapter 4. 4 (pgs 120 -123), and 20. 1 -2 • Problems in CTCS: 4. 37, 39 and 20. 3, 5, 7, 9

New Energy Sources – Why? • Run out of fossil fuels – Hydrocarbons not available for plastics or medicines • Fossil fuel wastes – CO 2 generation gives greenhouse – NOx and SOx give acid rain problems – Smog gives tropospheric ozone problems • CO and other particulates • Nuclear power wastes

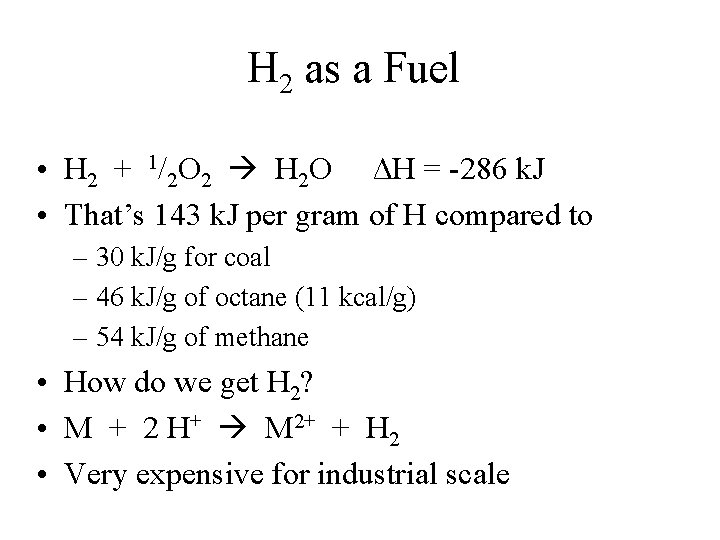
H 2 as a Fuel • H 2 + 1/2 O 2 H 2 O ΔH = -286 k. J • That’s 143 k. J per gram of H compared to – 30 k. J/g for coal – 46 k. J/g of octane (11 kcal/g) – 54 k. J/g of methane • How do we get H 2? • M + 2 H+ M 2+ + H 2 • Very expensive for industrial scale

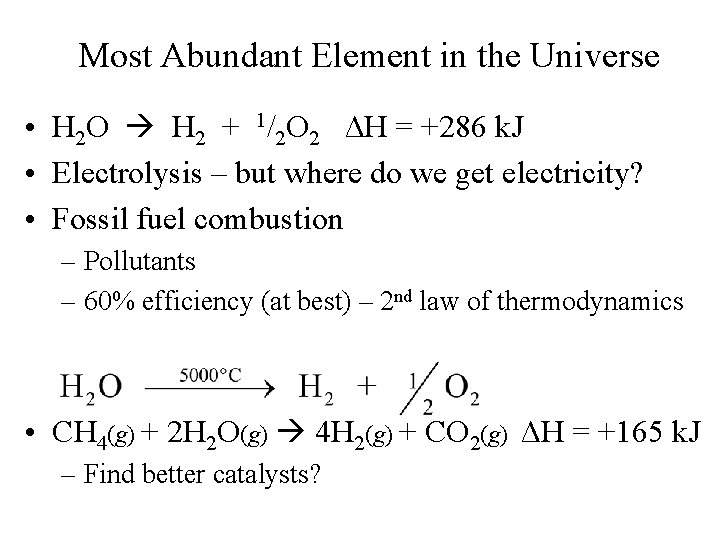
Most Abundant Element in the Universe • H 2 O H 2 + 1/2 O 2 ΔH = +286 k. J • Electrolysis – but where do we get electricity? • Fossil fuel combustion – Pollutants – 60% efficiency (at best) – 2 nd law of thermodynamics • CH 4(g) + 2 H 2 O(g) 4 H 2(g) + CO 2(g) ΔH = +165 k. J – Find better catalysts?

Storage and Combustion • 1 g of H 2 occupies 12 L of volume at atmospheric pressure • Condense it at -253ºC • Li(s) + 1/2 H 2(g) Li. H(s) – Occupies 1 teaspoon of volume • Li. H(s) + H 2 O(l) H 2(g) + Li. OH(s) • Hindenburg and space shuttle Challenger

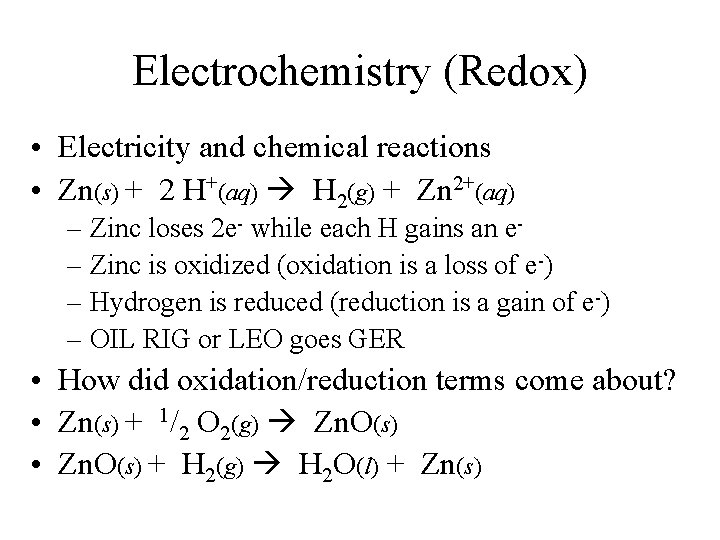
Electrochemistry (Redox) • Electricity and chemical reactions • Zn(s) + 2 H+(aq) H 2(g) + Zn 2+(aq) – Zinc loses 2 e- while each H gains an e– Zinc is oxidized (oxidation is a loss of e-) – Hydrogen is reduced (reduction is a gain of e-) – OIL RIG or LEO goes GER • How did oxidation/reduction terms come about? • Zn(s) + 1/2 O 2(g) Zn. O(s) • Zn. O(s) + H 2(g) H 2 O(l) + Zn(s)

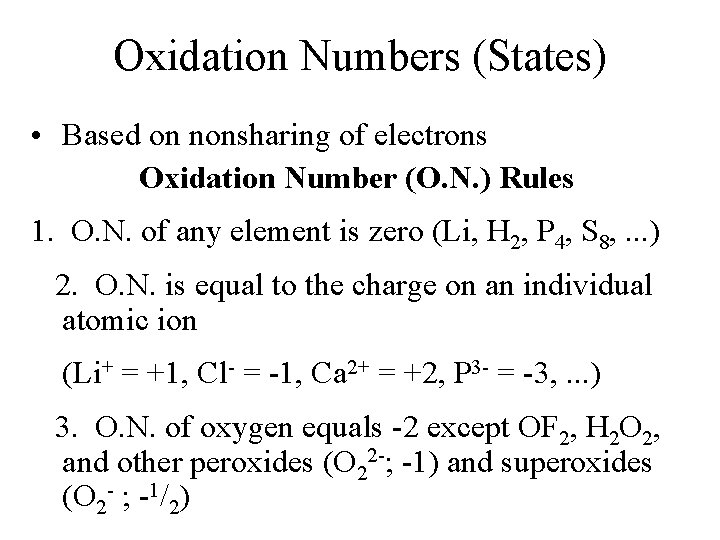
Oxidation Numbers (States) • Based on nonsharing of electrons Oxidation Number (O. N. ) Rules 1. O. N. of any element is zero (Li, H 2, P 4, S 8, . . . ) 2. O. N. is equal to the charge on an individual atomic ion (Li+ = +1, Cl- = -1, Ca 2+ = +2, P 3 - = -3, . . . ) 3. O. N. of oxygen equals -2 except OF 2, H 2 O 2, and other peroxides (O 22 -; -1) and superoxides (O 2 - ; -1/2)

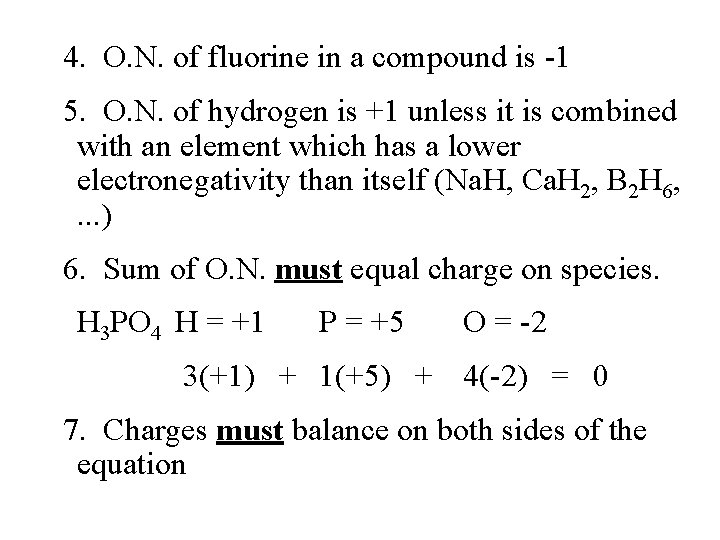
4. O. N. of fluorine in a compound is -1 5. O. N. of hydrogen is +1 unless it is combined with an element which has a lower electronegativity than itself (Na. H, Ca. H 2, B 2 H 6, . . . ) 6. Sum of O. N. must equal charge on species. H 3 PO 4 H = +1 P = +5 O = -2 3(+1) + 1(+5) + 4(-2) = 0 7. Charges must balance on both sides of the equation

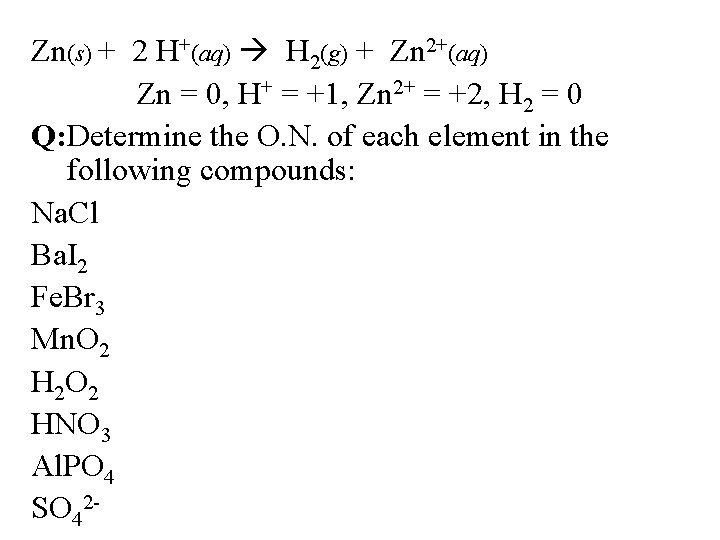
Zn(s) + 2 H+(aq) H 2(g) + Zn 2+(aq) Zn = 0, H+ = +1, Zn 2+ = +2, H 2 = 0 Q: Determine the O. N. of each element in the following compounds: Na. Cl Ba. I 2 Fe. Br 3 Mn. O 2 H 2 O 2 HNO 3 Al. PO 4 SO 42 -

Redox/Travel Agents • If one species is oxidized, another must be reduced (electrons have to go somewhere!) • The species that is oxidized is a reducing agent (oftentimes contains O) • The species that is reduced is an oxidizing agent Zn(s) + 2 H+(aq) H 2(g) + Zn 2+(aq) Q: Which is the reducing agent and which is the oxidizing agent?

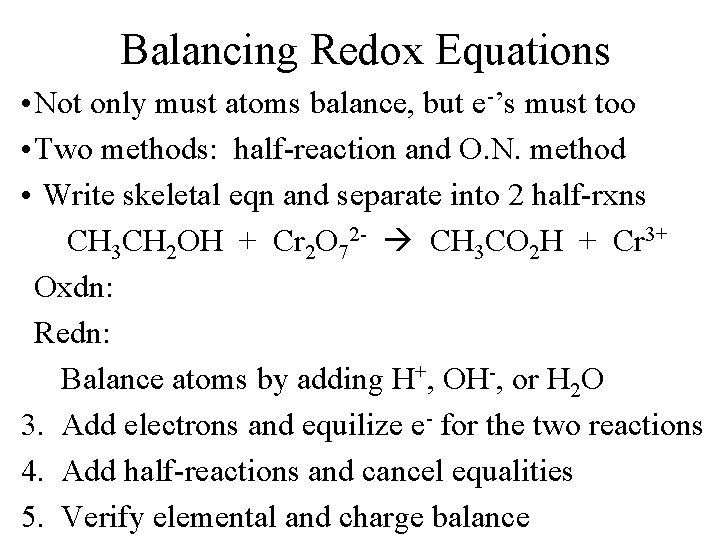
Balancing Redox Equations • Not only must atoms balance, but e-’s must too • Two methods: half-reaction and O. N. method • Write skeletal eqn and separate into 2 half-rxns CH 3 CH 2 OH + Cr 2 O 72 - CH 3 CO 2 H + Cr 3+ Oxdn: Redn: Balance atoms by adding H+, OH-, or H 2 O 3. Add electrons and equilize e- for the two reactions 4. Add half-reactions and cancel equalities 5. Verify elemental and charge balance

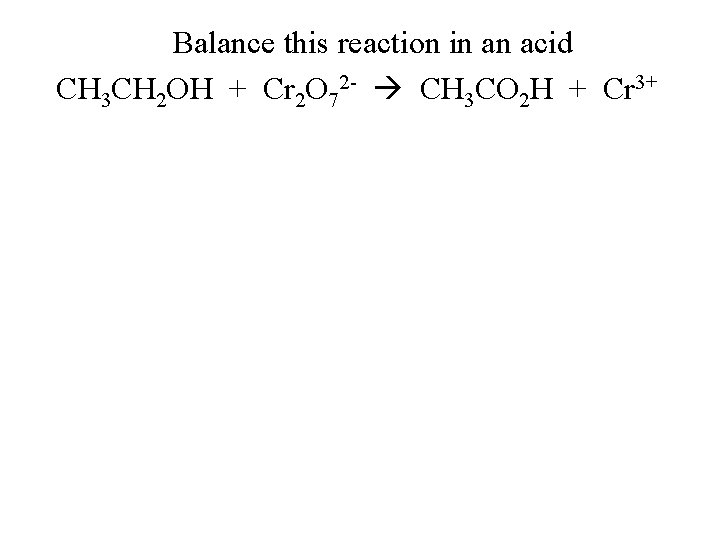
Balance this reaction in an acid CH 3 CH 2 OH + Cr 2 O 72 - CH 3 CO 2 H + Cr 3+

• In a basic solution, Br 2, disproportionates to bromate (Br. O 3 -) and bromide ions. Balance this equation.

• In a basic solution, iron(III) hydroxide reacts with hypochlorite (OCl-) ion to produce Fe. O 42 and chloride. Balance this reaction.