Chapter 2 CIC and Chapter 8 CTCS Read







- Slides: 14

Chapter 2 (CIC) and Chapter 8 (CTCS) • Read in CTCS Chapter 8. 1, 4, 6 -8 • Problems in CTCS: 3, 31, 33, 47 abcdf, 49 bd, 51 a, 59 bd, 61

Ozone • A pollutant in troposphere • A filter of UV light in stratosphere • 3 O 2(g) + Energy 2 O 3(g) 4 Energy = lightning, photocopier, electric arc, etc. • Allotrope (e. g. , graphite, diamond, fullerene) • Used for bleaching (wood, fabric, water) • Why is ozone different from oxygen and why is it helpful in one part of our atmosphere but not in another?

Periodic Table • Why is periodic table laid out the way it is? • Li, Na, K demo • Valence electrons (e-) – account for chemical and physical properties of elements and are the outermost electrons of an atom • Can be determined for any representative element by the number above the Group (Family) in the periodic table (1 A-8 A) • Families: Alkali metal, Alkaline Earth, Chalcogen, Halogen, Noble Gas

Getting to Low Energy • Noble Gas – “inert” because it is stable by itself • These elements have 8 valence e- (except He) • Other elements try to attain this low energy state as well • Elements do this by gaining, losing, or sharing e - • Sharing of e- yields covalent bonds

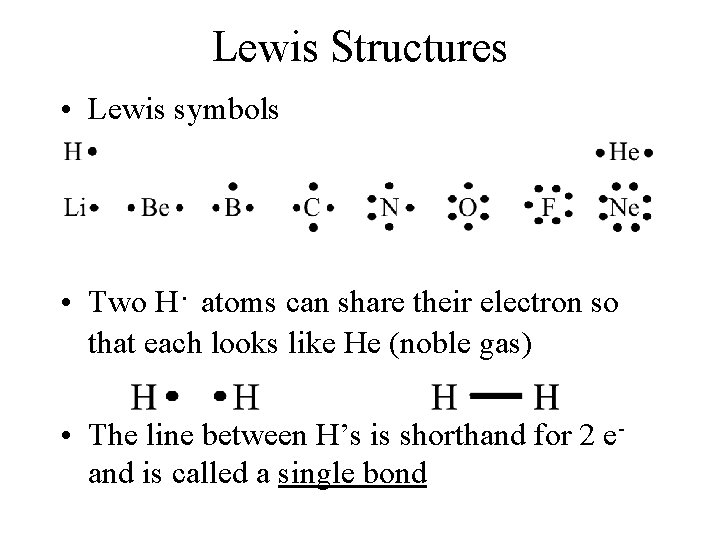
Lewis Structures • Lewis symbols • Two H· atoms can share their electron so that each looks like He (noble gas) • The line between H’s is shorthand for 2 eand is called a single bond

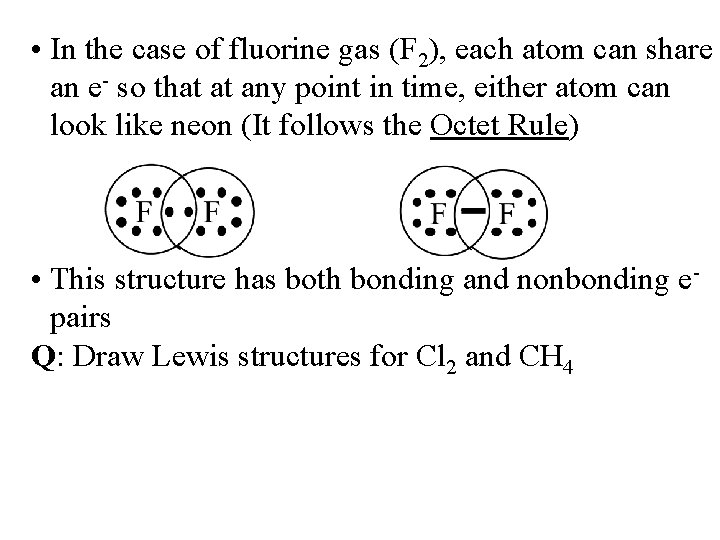
• In the case of fluorine gas (F 2), each atom can share an e- so that at any point in time, either atom can look like neon (It follows the Octet Rule) • This structure has both bonding and nonbonding epairs Q: Draw Lewis structures for Cl 2 and CH 4

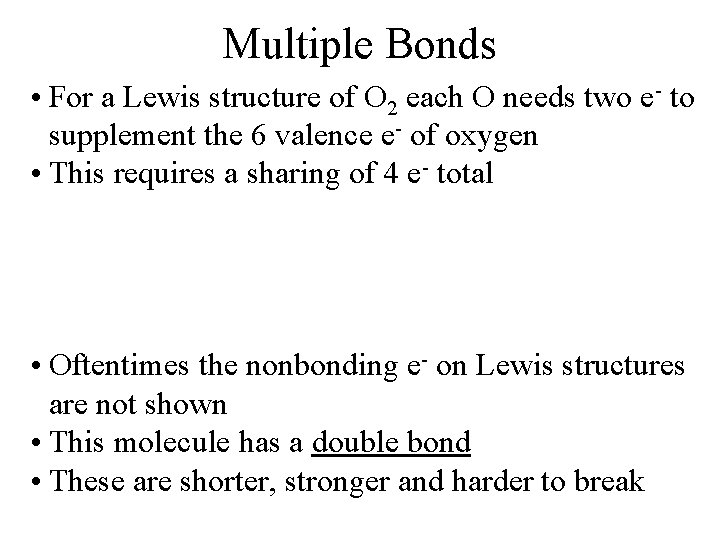
Multiple Bonds • For a Lewis structure of O 2 each O needs two e- to supplement the 6 valence e- of oxygen • This requires a sharing of 4 e- total • Oftentimes the nonbonding e- on Lewis structures are not shown • This molecule has a double bond • These are shorter, stronger and harder to break

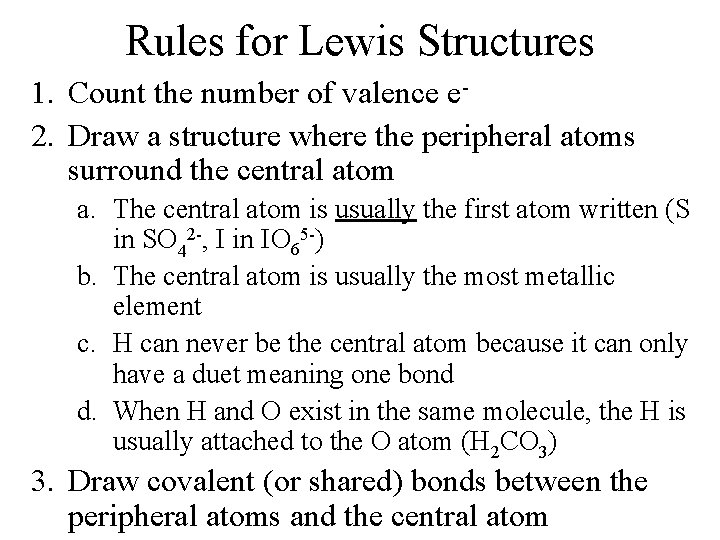
Rules for Lewis Structures 1. Count the number of valence e 2. Draw a structure where the peripheral atoms surround the central atom a. The central atom is usually the first atom written (S in SO 42 -, I in IO 65 -) b. The central atom is usually the most metallic element c. H can never be the central atom because it can only have a duet meaning one bond d. When H and O exist in the same molecule, the H is usually attached to the O atom (H 2 CO 3) 3. Draw covalent (or shared) bonds between the peripheral atoms and the central atom

4. Determine the number of valence e- still available 5. Fill the octets for the peripheral atoms. 6. Fill the octet for the central atom (if there are enough valence e-) 7. If there are not enough e- to accomplish #6, make multiple bonds between the central and peripheral atoms You should check two things when finished: 1. Are you showing the correct number of valence e -? 2. Does each atom have an octet?

Draw Lewis Structures for CO 2, N 2, CO, PCl 3, CH 3 OH and H 2 CO It doesn’t matter what the bond angles are

The structure of O 3 1. 3 x 6 = 18 e 2, 3. O-O-O 4. 18 - 4 = 14 e 5. 6. 7. *These are not linear molecules

Resonance • These two structures are averaged together to get the “actual” structure and they are referred to as resonance structures • The actual structure has an O-O bond length of 1. 28Å compared to an O-O typically at 1. 47Å and O=O at 1. 21Å • Resonance structures only move e-, not atoms

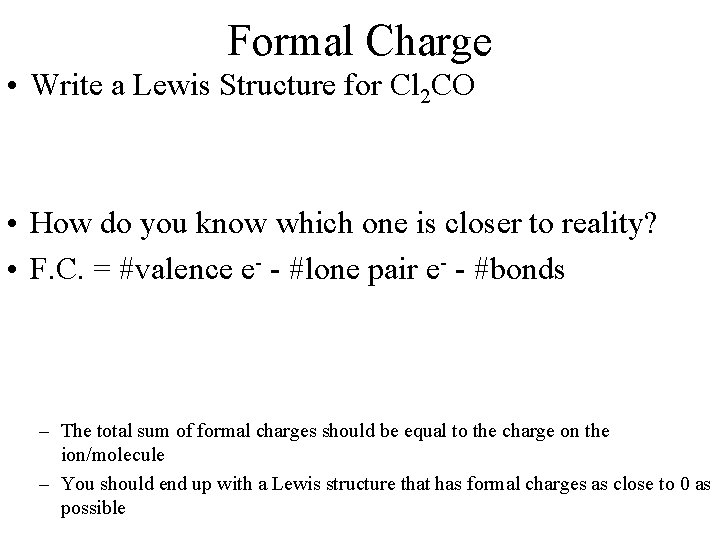
Formal Charge • Write a Lewis Structure for Cl 2 CO • How do you know which one is closer to reality? • F. C. = #valence e- - #lone pair e- - #bonds – The total sum of formal charges should be equal to the charge on the ion/molecule – You should end up with a Lewis structure that has formal charges as close to 0 as possible

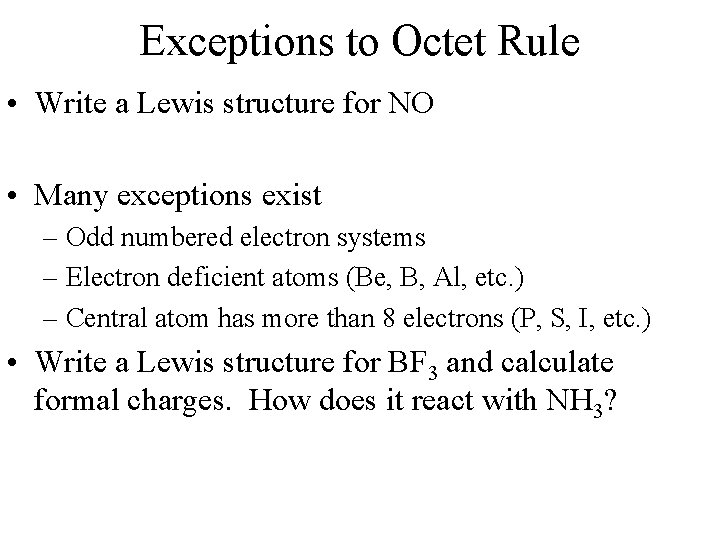
Exceptions to Octet Rule • Write a Lewis structure for NO • Many exceptions exist – Odd numbered electron systems – Electron deficient atoms (Be, B, Al, etc. ) – Central atom has more than 8 electrons (P, S, I, etc. ) • Write a Lewis structure for BF 3 and calculate formal charges. How does it react with NH 3?