Chapter 12 Section 1 Arranging the Elements The





- Slides: 9

Chapter 12 Section 1 Arranging the Elements The Periodic Table Crash Course --- The Periodic Table

Why arrange the elements? • Read page 336 • Why would grouping elements by their properties help scientist? • So they could understand how the elements interact with one another. • What helped Mendeleev see the pattern of the elements? • He arranged them in order of increasing atomic mass (modern day by atomic number)

Read p. 337 • What was seen when Mendeleev arranged the elements in order of increasing mass? • He saw a repeating pattern • What is the name of something that occurs or repeats at regular intervals? • Periodic • Who worked with the atomic number (number of protons) in atoms • Henry Moseley

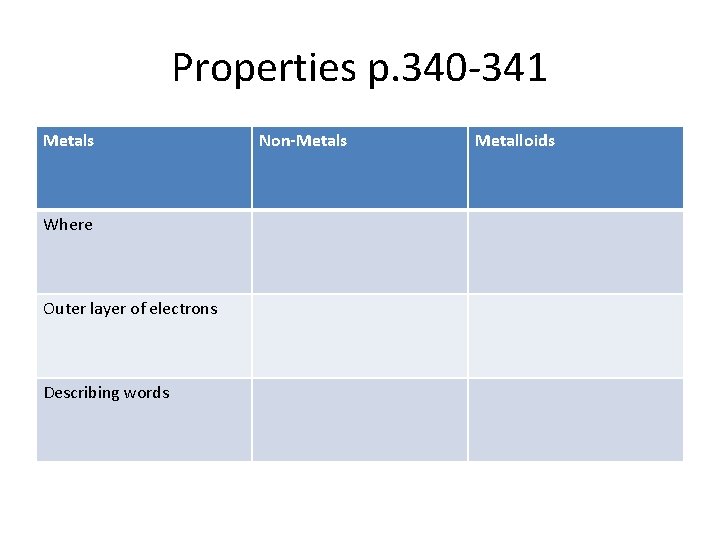
Properties p. 340 -341 Metals Where Outer layer of electrons Describing words Non-Metals Metalloids

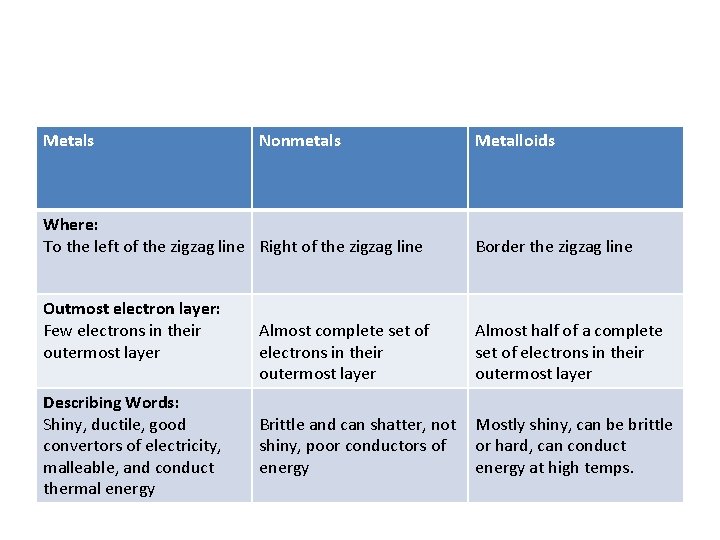
Metals Nonmetals Where: To the left of the zigzag line Right of the zigzag line Outmost electron layer: Few electrons in their outermost layer Describing Words: Shiny, ductile, good convertors of electricity, malleable, and conduct thermal energy Metalloids Border the zigzag line Almost complete set of electrons in their outermost layer Almost half of a complete set of electrons in their outermost layer Brittle and can shatter, not shiny, poor conductors of energy Mostly shiny, can be brittle or hard, can conduct energy at high temps.

Decoding the periodic table Read p. 342 What does a chemical symbol do? Identify the element How are chemical symbols written? With a capital letter and a lower case letter for the second letter if needed • New elements are written how? • With temporary 3 letter symbols • • •

Periods • What is a period in chemistry? • A horizontal row of elements in the periodic table • The physical and chemical properties in these rows follow what type of pattern? • repeating

Columns • What is a group? • A vertical column of elements in the periodic table; elements in a group share chemical properties • Why is a group sometimes called a family? • Often, they have similar chemical and physical properties • Bozerman Science --- A Tour of the Periodic Table

Exit Slip • On a slip of paper, answer the following questions: • 1. How are the elements arranged in the modern periodic table? • 2. A horizontal row of elements in the periodic table. • 3. A column of elements is known as a family or a _____.