Cell potentials and Reduction potentials The light at





- Slides: 6

Cell potentials and Reduction potentials

The light at the end of the tunnel Read 17. 7 (pg. 716), do PE 7, 8 - use Ex. 17. 8 (ignore Ex. 17. 7). Also 17. 62, 17. 64 (731) Note: you need to multiply equations so that e– cancel out. However, unlike Hess’s law problems, DO NOT also multiply E° PE 7 - Ni. O 2 has the greater reduction potential, thus it is reduced and Fe is oxidized … Ni. O 2 + 2 H 2 O + 2 e– Ni(OH)2 + 2 OH– Fe + 2 OH– Fe(OH)2 + 2 e– Ni. O 2 + 2 H 2 O + Fe Ni(OH)2 + Fe(OH)2 E°cell = E°reduced - E°oxidized = 0. 49 V - -0. 88 V = 1. 37 V

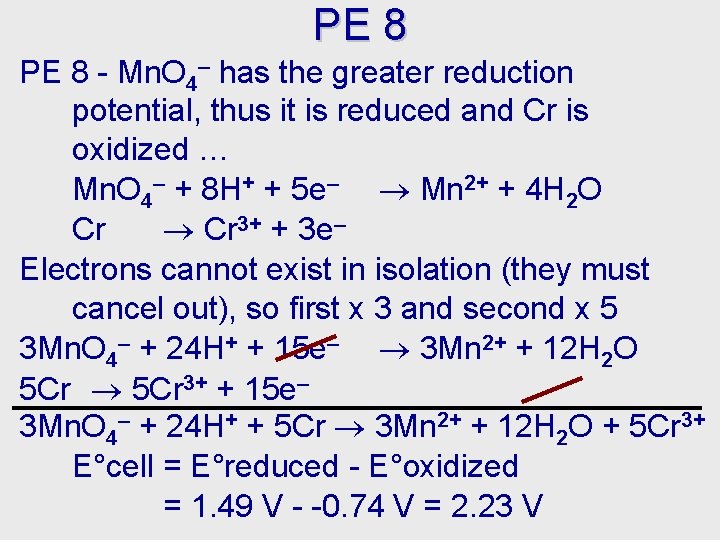
PE 8 - Mn. O 4– has the greater reduction potential, thus it is reduced and Cr is oxidized … Mn. O 4– + 8 H+ + 5 e– Mn 2+ + 4 H 2 O Cr 3+ + 3 e– Electrons cannot exist in isolation (they must cancel out), so first x 3 and second x 5 3 Mn. O 4– + 24 H+ + 15 e– 3 Mn 2+ + 12 H 2 O 5 Cr 3+ + 15 e– 3 Mn. O 4– + 24 H+ + 5 Cr 3 Mn 2+ + 12 H 2 O + 5 Cr 3+ E°cell = E°reduced - E°oxidized = 1. 49 V - -0. 74 V = 2. 23 V

17. 62 We’re looking for half cells that contain: NO 3– … NO … and Fe 2+ Fe 3+ In table 17. 1 we find: NO 3– + 4 H+ + 3 e– NO + 2 H 2 O E° = 0. 96 V Fe 2+ Fe 3+ + e– E° = 0. 77 V NO 3– + 4 H+ + 3 e– NO + 2 H 2 O E° = 0. 96 V 3 Fe 2+ 3 Fe 3+ + 3 e– E° = 0. 77 V NO 3– + 4 H+ 3 Fe 2+ 3 Fe 3+ + NO + 2 H 2 O E°cell = E°reduced - E°oxidized = 0. 96 V - 0. 77 V = 0. 19 V

17. 64 Br. O 3– has the greater reduction potential, thus it is reduced and I– is oxidized … Br. O 3– + 6 H+ + 6 e– Br– + 3 H 2 O 2 I– I 2 + 2 e– Electrons cannot exist in isolation (they must cancel out), so second x 3 Br. O 3– + 6 H+ + 6 e– Br– + 3 H 2 O 6 I– 3 I 2 + 6 e– Br. O 3– + 6 H+ + 6 I– Br– + 3 H 2 O + 3 I 2 E°cell = E°reduced - E°oxidized = 1. 44 V - 0. 54 V = 0. 90 V

The End For more lessons, visit www. chalkbored. com