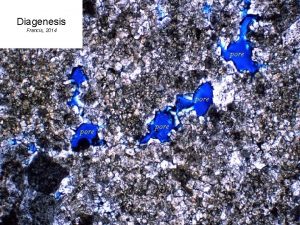
Biogenic opal diagenesis in sediments Biogenic opal What

Biogenic opal diagenesis in sediments
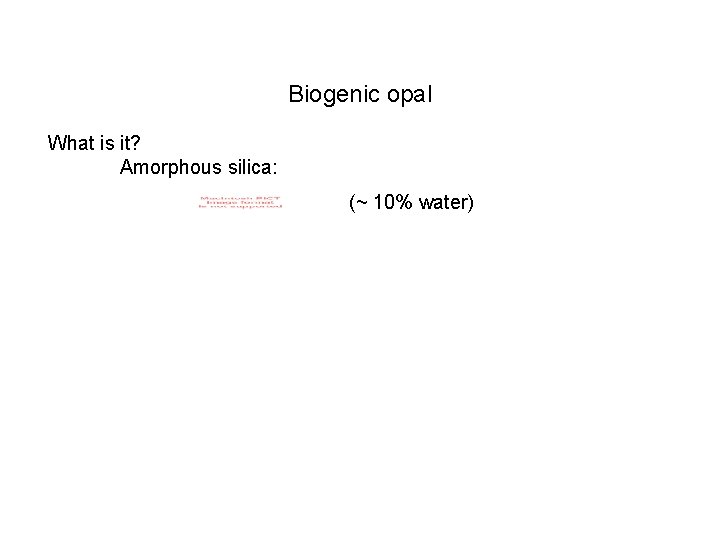
Biogenic opal What is it? Amorphous silica: (~ 10% water)
Biogenic opal What is it? Amorphous silica: (~ 10% water) Measurement: Leach solid in Na 2 CO 3 ; make a correction for detrital Si
Biogenic opal What is it? Amorphous silica: (~ 10% water) Precipitated in the surface ocean by: -- phytoplankton diatoms, silicoflagellates -- protozoans radiolaria
Biogenic opal What is it? Amorphous silica: (~ 10% water) Precipitated in the surface ocean by: -- phytoplankton diatoms, silicoflagellates -- protozoans radiolaria A fraction fall of this opal falls to the sea floor -- it’s efficiently recycled, in water column and sediments -- overall, ~ 3% of opal production is preserved in sediments
Biogenic opal What is it? Amorphous silica: (~ 10% water) Biogenic opal is soluble in seawater, Si(OH)4 is a weak acid, p. K ~9 ==> it’s mostly protonated in seawater
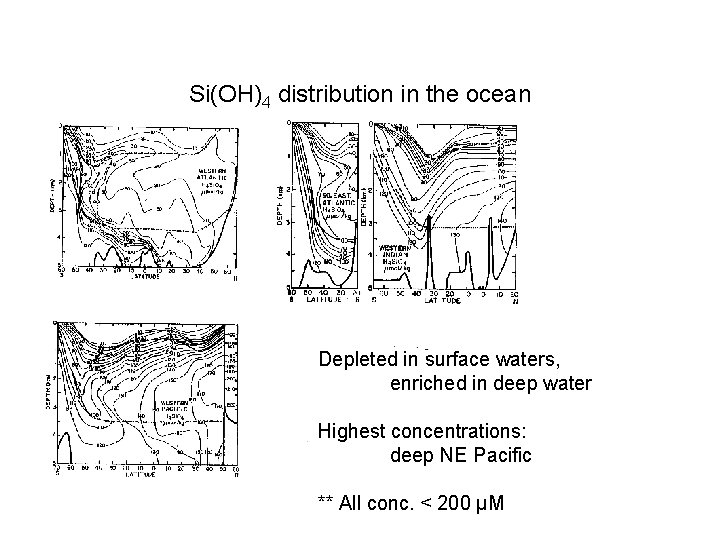
Si(OH)4 distribution in the ocean Depleted in surface waters, enriched in deep water Highest concentrations: deep NE Pacific ** All conc. < 200 µM

The solubility of biogenic opal in seawater Initial Studies -- Hurd, 1973, GCA 37, 2257 -2282 Experiment: -- separate opal from cores -- clean with acid -- place in sw at controlled temp After ~ days:
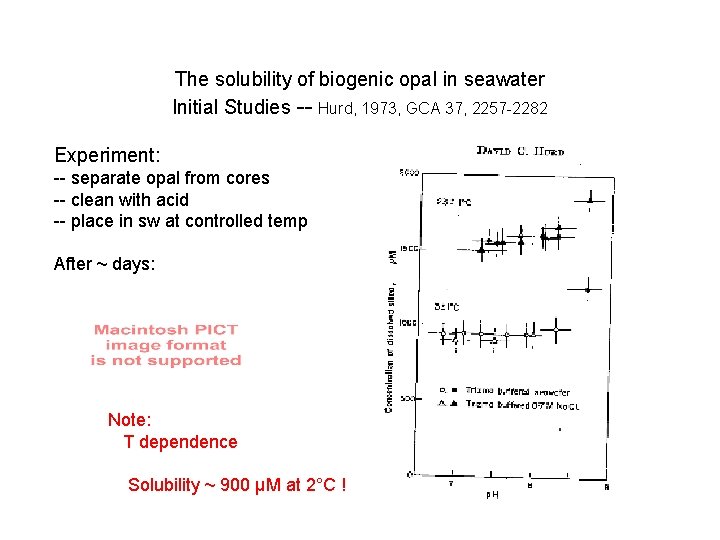
The solubility of biogenic opal in seawater Initial Studies -- Hurd, 1973, GCA 37, 2257 -2282 Experiment: -- separate opal from cores -- clean with acid -- place in sw at controlled temp After ~ days: Note: T dependence Solubility ~ 900 µM at 2°C !
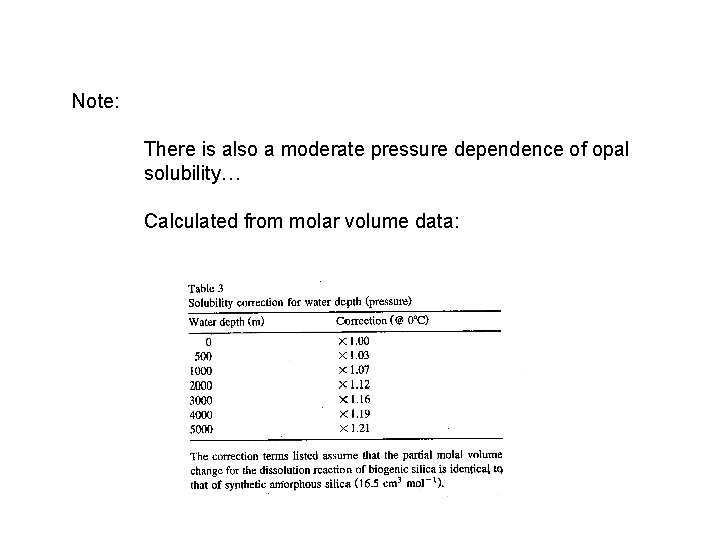
Note: There is also a moderate pressure dependence of opal solubility… Calculated from molar volume data:
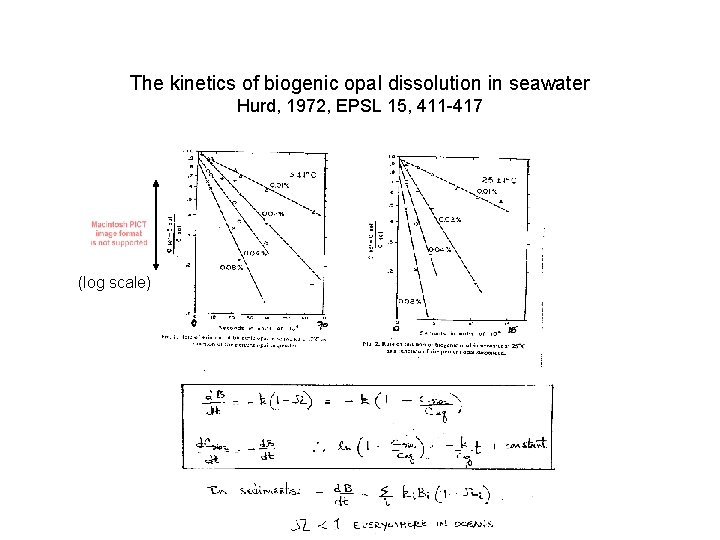
The kinetics of biogenic opal dissolution in seawater Hurd, 1972, EPSL 15, 411 -417 (log scale)
![A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4] A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4]](http://slidetodoc.com/presentation_image/794d59e1e124c1a086c1309f782e2a28/image-12.jpg)
A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4] in pore water to look like?
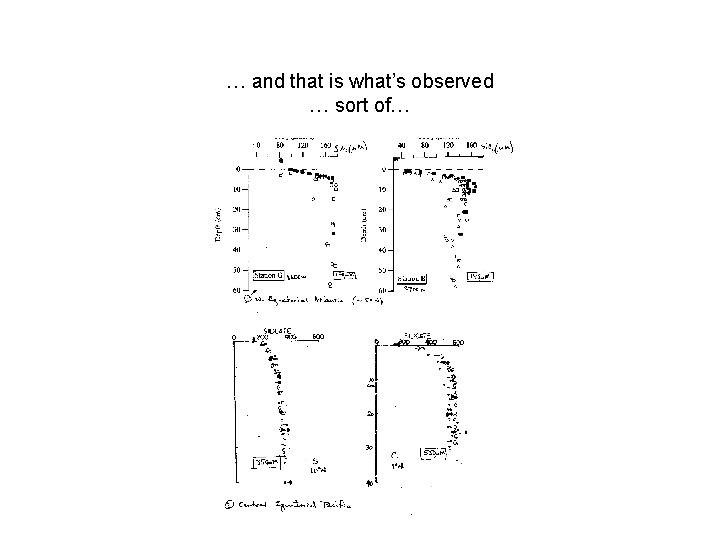
… and that is what’s observed … sort of…
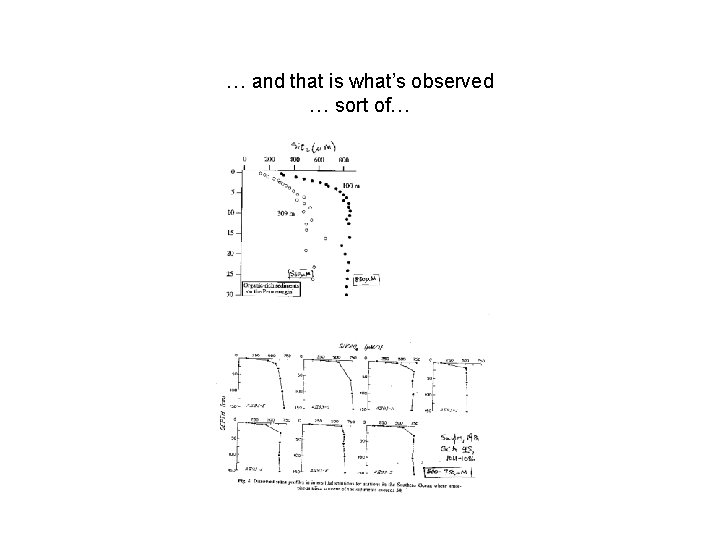
… and that is what’s observed … sort of…
![Comparing asymptotic pore water [Si(OH)4] to the “equilibrium” value Comparing asymptotic pore water [Si(OH)4] to the “equilibrium” value](http://slidetodoc.com/presentation_image/794d59e1e124c1a086c1309f782e2a28/image-15.jpg)
Comparing asymptotic pore water [Si(OH)4] to the “equilibrium” value
Explaining the discrepancy between the aymptotic and apparent equilibrium values… A rate law for opal dissolution:
Explaining the discrepancy between the aymptotic and apparent equilibrium values… A rate law for opal dissolution: Explanation I : It’s a kinetic phenomenon: ki. Bi --> 0 with increasing depth in the sediments

Explaining the discrepancy between the aymptotic and apparent equilibrium values… A rate law for opal dissolution: Explanation II : Solubility (Cequil) varies from place to place Proposal: a) Opal solubility depends on Al/Si in the opal b) Al is released by minerals during diagenesis, then substitutes for Si in opal

Explaining the discrepancy between the aymptotic and apparent equilibrium values… A rate law for opal dissolution: Explanation III: The rate law above is incomplete… In addition, there is reprecipitation of Si(OH)4 as authigenic aluminosilicate
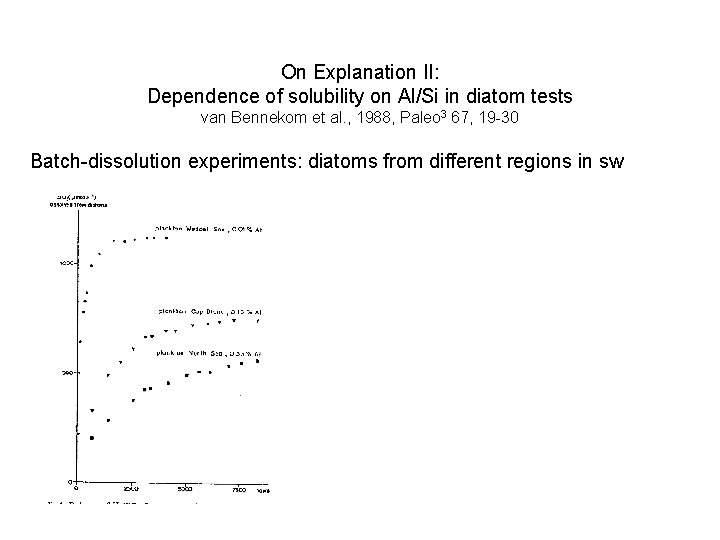
On Explanation II: Dependence of solubility on Al/Si in diatom tests van Bennekom et al. , 1988, Paleo 3 67, 19 -30 Batch-dissolution experiments: diatoms from different regions in sw
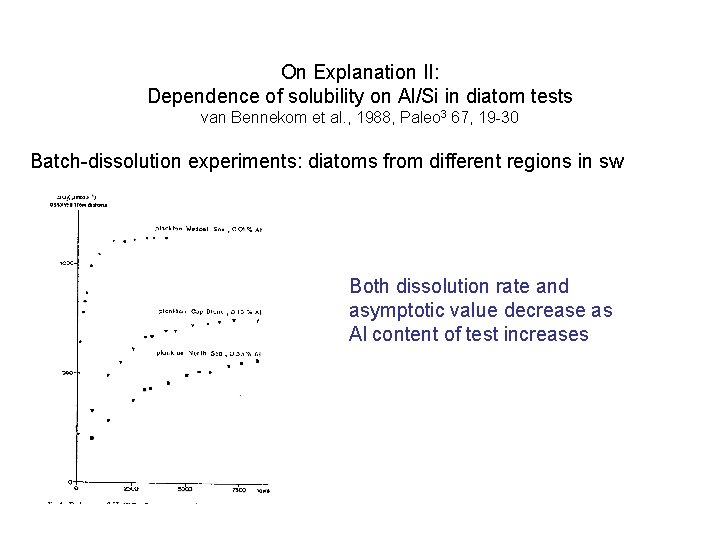
On Explanation II: Dependence of solubility on Al/Si in diatom tests van Bennekom et al. , 1988, Paleo 3 67, 19 -30 Batch-dissolution experiments: diatoms from different regions in sw Both dissolution rate and asymptotic value decrease as Al content of test increases
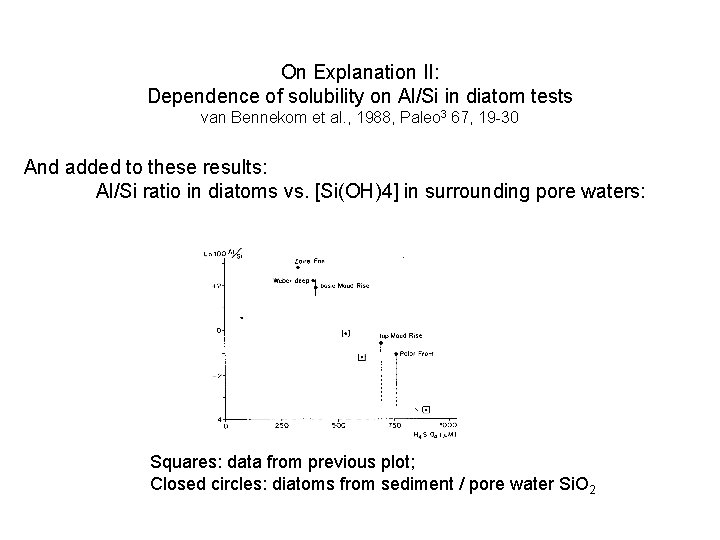
On Explanation II: Dependence of solubility on Al/Si in diatom tests van Bennekom et al. , 1988, Paleo 3 67, 19 -30 And added to these results: Al/Si ratio in diatoms vs. [Si(OH)4] in surrounding pore waters: Squares: data from previous plot; Closed circles: diatoms from sediment / pore water Si. O 2
A study of opal diagenesis in the Southern Ocean (Indian Ocean sector) van Cappellen and Qiu, 1997, DSRII 44, 1109 -1128 Dixit et al. , 2001, Mar Chem 73, 333 -352
A study of opal diagenesis in the Southern Ocean (Indian Ocean sector) van Cappellen and Qiu, 1997, DSRII 44, 1109 -1128 Dixit et al. , 2001, Mar Chem 73, 333 -352
A study of opal diagenesis in the Southern Ocean (Indian Ocean sector) van Cappellen and Qiu, 1997, DSRII 44, 1109 -1128 Dixit et al. , 2001, Mar Chem 73, 333 -352
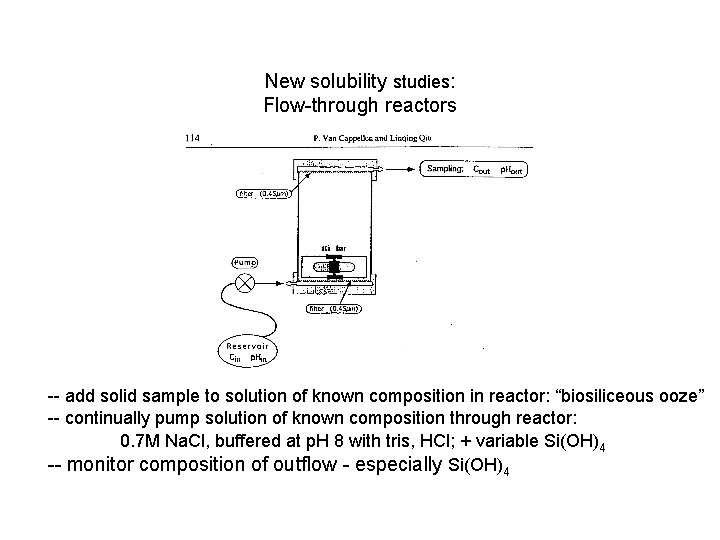
New solubility studies: Flow-through reactors -- add solid sample to solution of known composition in reactor: “biosiliceous ooze” -- continually pump solution of known composition through reactor: 0. 7 M Na. Cl, buffered at p. H 8 with tris, HCl; + variable Si(OH)4 -- monitor composition of outflow - especially Si(OH)4
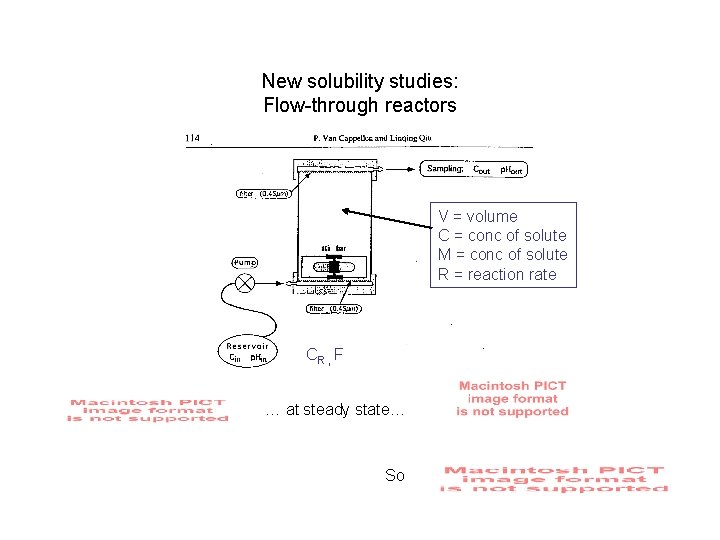
New solubility studies: Flow-through reactors V = volume C = conc of solute M = conc of solute R = reaction rate CR , F … at steady state… So
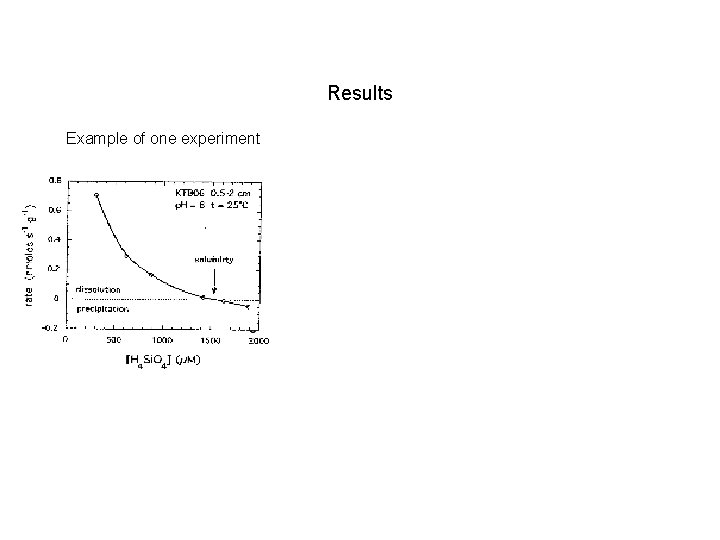
Results Example of one experiment
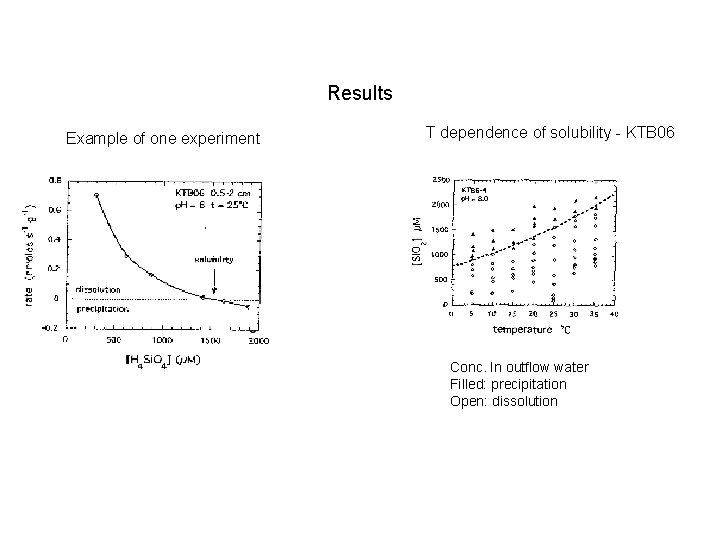
Results Example of one experiment T dependence of solubility - KTB 06 Conc. In outflow water Filled: precipitation Open: dissolution
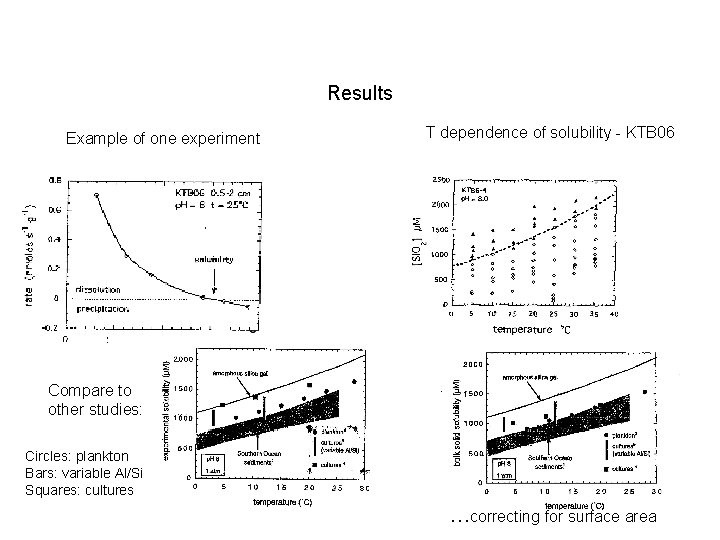
Results Example of one experiment T dependence of solubility - KTB 06 Compare to other studies: Circles: plankton Bars: variable Al/Si Squares: cultures …correcting for surface area
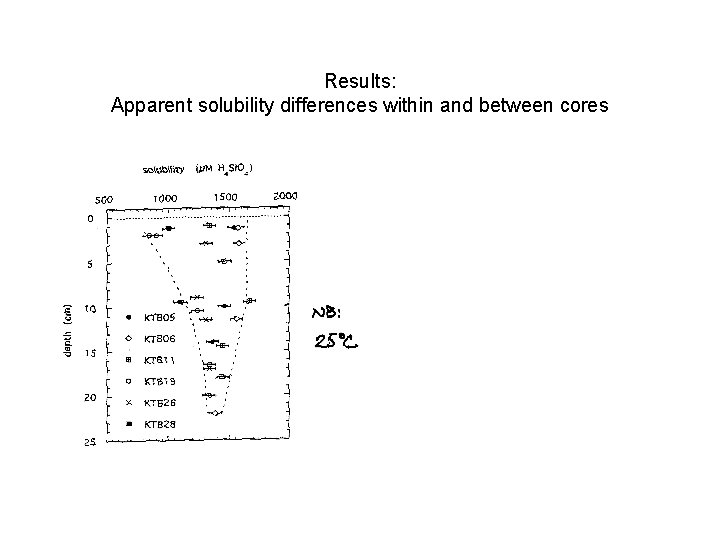
Results: Apparent solubility differences within and between cores
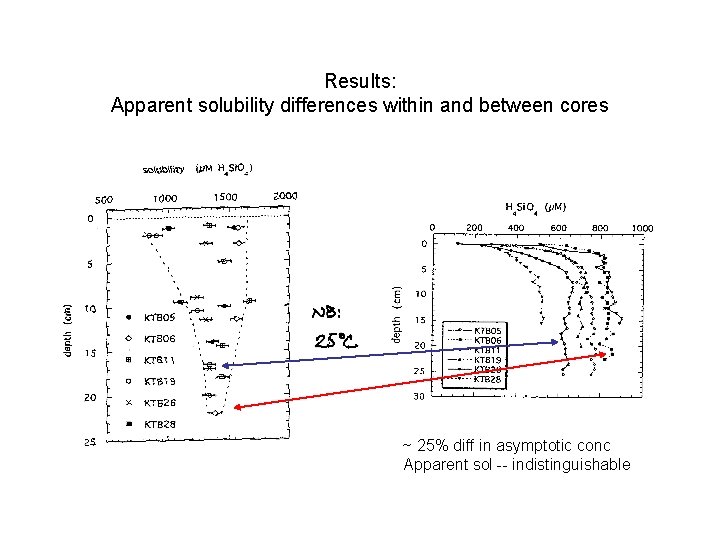
Results: Apparent solubility differences within and between cores ~ 25% diff in asymptotic conc Apparent sol -- indistinguishable
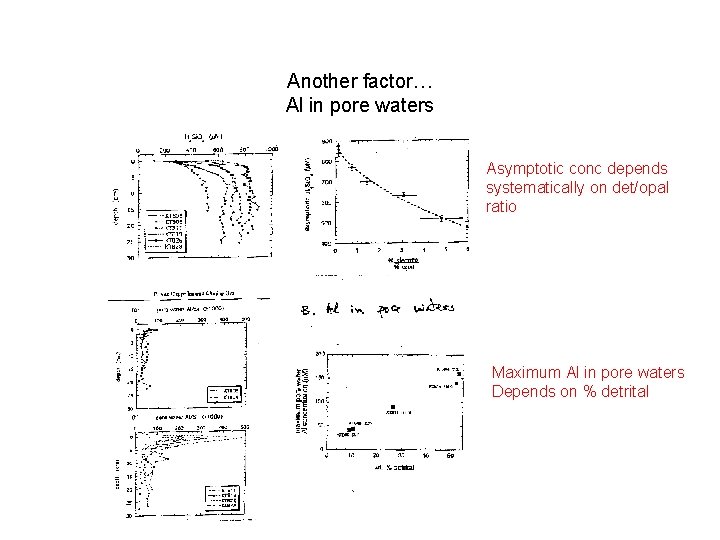
Another factor… Al in pore waters Asymptotic conc depends systematically on det/opal ratio Maximum Al in pore waters Depends on % detrital
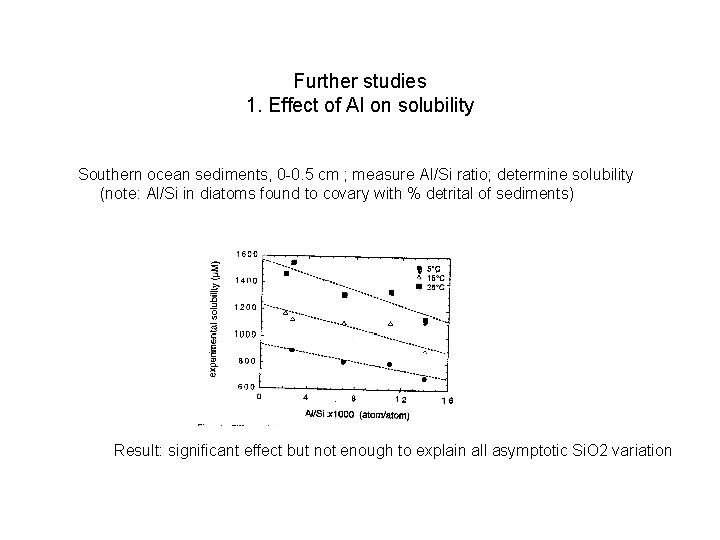
Further studies 1. Effect of Al on solubility Southern ocean sediments, 0 -0. 5 cm ; measure Al/Si ratio; determine solubility (note: Al/Si in diatoms found to covary with % detrital of sediments) Result: significant effect but not enough to explain all asymptotic Si. O 2 variation
Further studies 2. Experiment on effect of %det/%opal on equilibrium Si(OH)4 Mix detrital material and biogenic opal in variable proportions; observe the dissolved Si(OH)4 At steady state Detrital material: (a) kaolinite -- Al 2 Si 2(OH)4 (b) basalt (48. 5 wt% Si. O 2 ; 14. 4 wt % Al 2 O 3 21 -month batch experiments
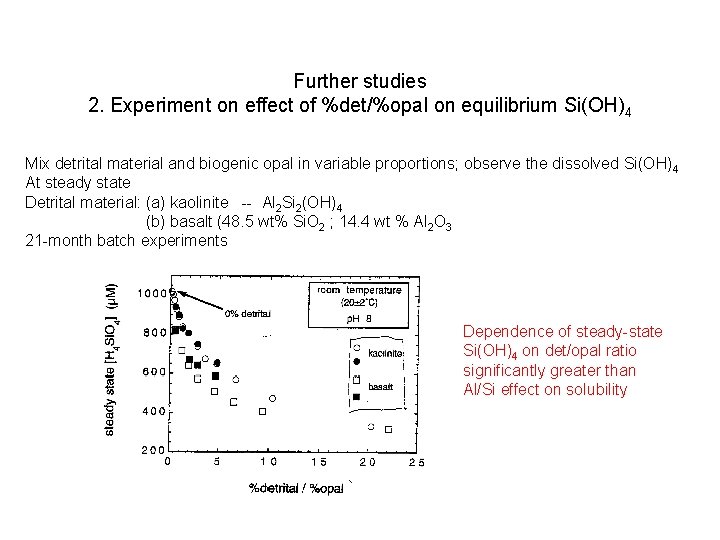
Further studies 2. Experiment on effect of %det/%opal on equilibrium Si(OH)4 Mix detrital material and biogenic opal in variable proportions; observe the dissolved Si(OH)4 At steady state Detrital material: (a) kaolinite -- Al 2 Si 2(OH)4 (b) basalt (48. 5 wt% Si. O 2 ; 14. 4 wt % Al 2 O 3 21 -month batch experiments Dependence of steady-state Si(OH)4 on det/opal ratio significantly greater than Al/Si effect on solubility

Further studies 3. Are authigenic aluminosilicates precipitating? Experiment: flow through reactors, containting 50 mg “biosiliceous ooze” Inflow: 500 n. M Al, variable Si(OH)4
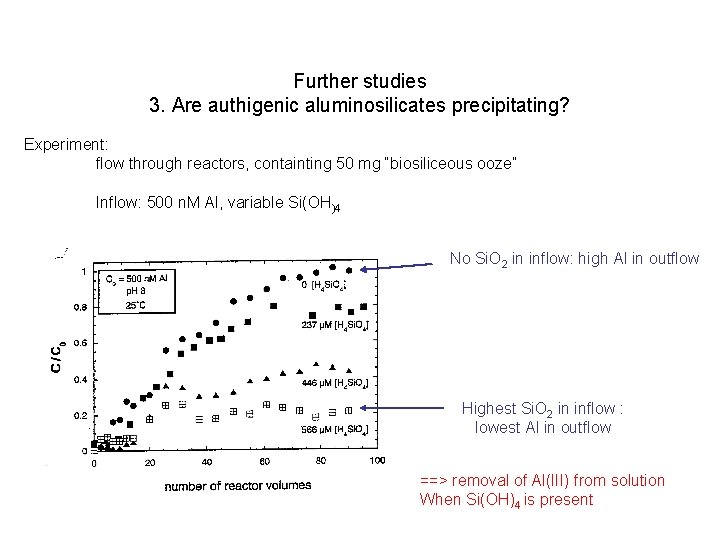
Further studies 3. Are authigenic aluminosilicates precipitating? Experiment: flow through reactors, containting 50 mg “biosiliceous ooze” Inflow: 500 n. M Al, variable Si(OH)4 No Si. O 2 in inflow: high Al in outflow Highest Si. O 2 in inflow : lowest Al in outflow ==> removal of Al(III) from solution When Si(OH)4 is present
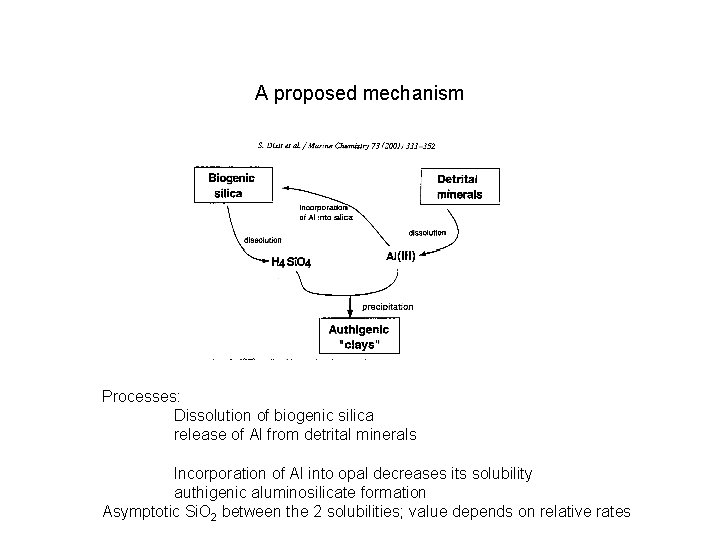
A proposed mechanism Processes: Dissolution of biogenic silica release of Al from detrital minerals Incorporation of Al into opal decreases its solubility authigenic aluminosilicate formation Asymptotic Si. O 2 between the 2 solubilities; value depends on relative rates
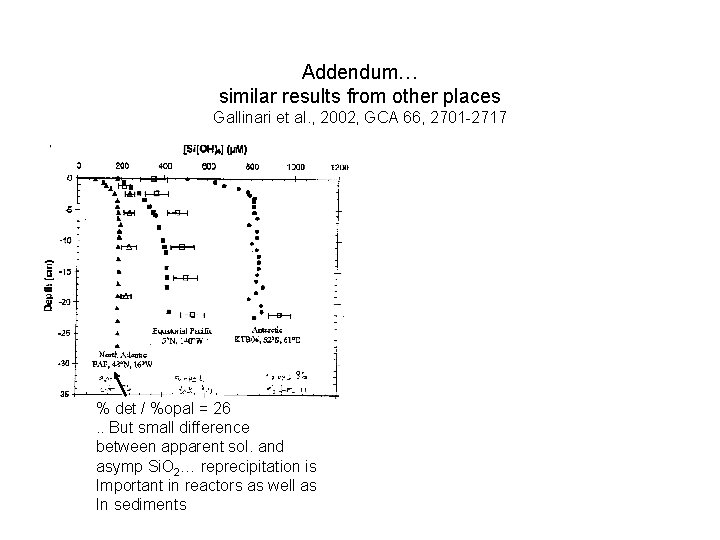
Addendum… similar results from other places Gallinari et al. , 2002, GCA 66, 2701 -2717 % det / %opal = 26. . But small difference between apparent sol. and asymp Si. O 2… reprecipitation is Important in reactors as well as In sediments
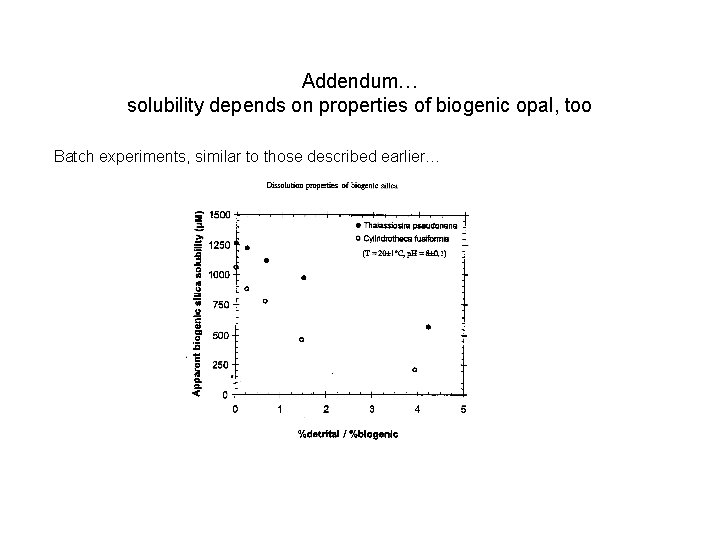
Addendum… solubility depends on properties of biogenic opal, too Batch experiments, similar to those described earlier…
- Slides: 41