As always n n OWL LonCapa assignments Lecture





- Slides: 11

As always… n n OWL Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

Clicker Question Consider a compound formed from a Group 2 element (such as Be or Mg and designated as M below) and a Group 6 element (such as O or S and designated as X below). Which of the following is the most reasonable statement? a) The compound between M and X will be in a 1: 1 ratio but it is best to think of the bond as polar covalent since atoms do not “want” to lose electrons (thermodynamically speaking). b) The bond between M and X is polar covalent as explained in “a” above, but we cannot determine the molecular formula without a molar mass. c) An ionic compound with the ions M 2+ and X 2 - is the only stable form of the compound. d) An ionic compound with the ions M+ and X- could be thermodynamically favorable, but an ionic compound with the ions M 2+ and X 2 - is more thermodynamically favorable. e) An ionic compound will form but we cannot predict the charges with any degree of accuracy. 2

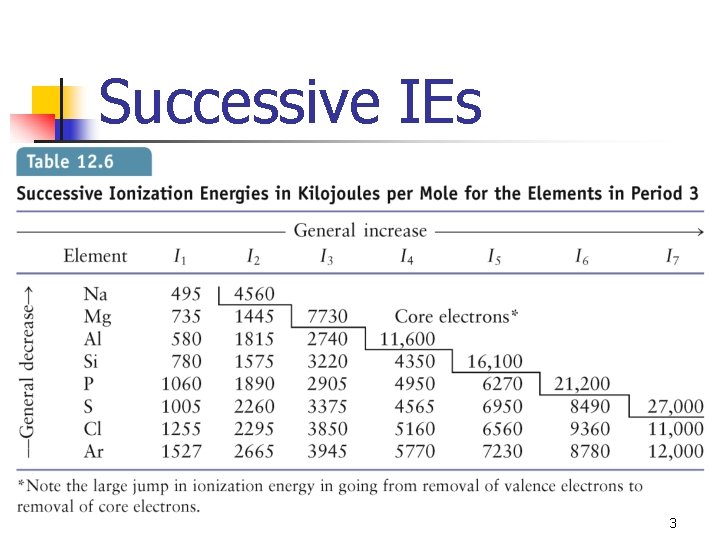
Successive IEs 3

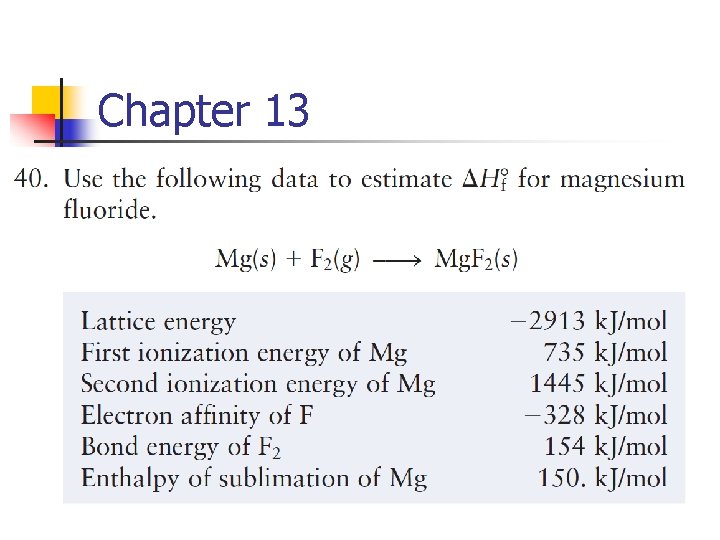
Chapter 13 4

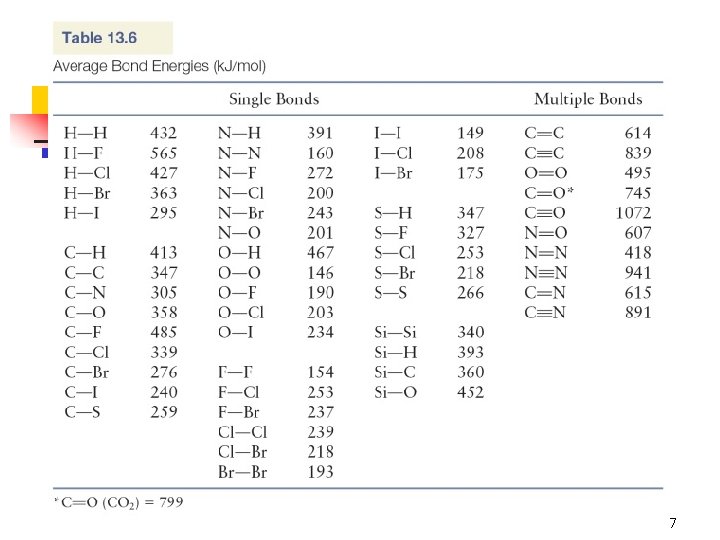
Chapter 13 (OWL) 135. Given the following information: Heat of sublimation of Li(s) = 166 k. J/mol Bond energy of HCl = 427 k. J/mol Ionization energy of Li(g) = 520. k. J/mol Electron affinity of Cl(g) = – 349 k. J/mol Lattice energy of Li. Cl(s) = – 829 k. J/mol Bond energy of H 2 = 432 k. J/mol Calculate the net change in energy for the following reaction: 2 Li(s) + 2 HCl(g) → 2 Li. Cl(s) + H 2(g) 5

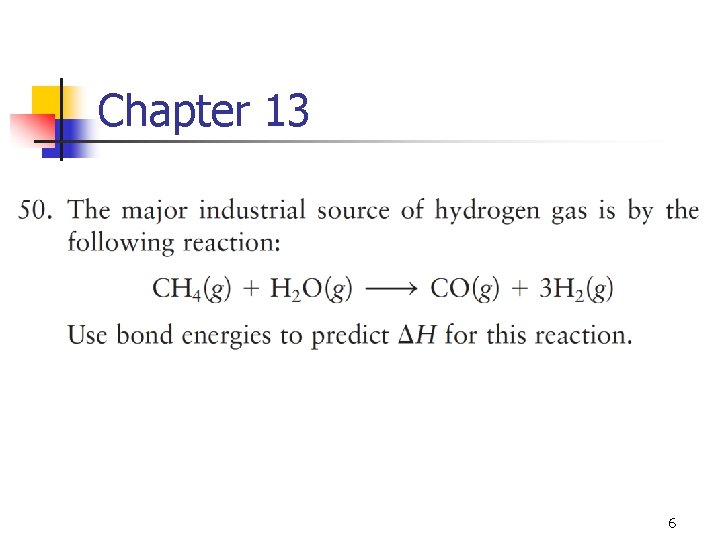
Chapter 13 6

7


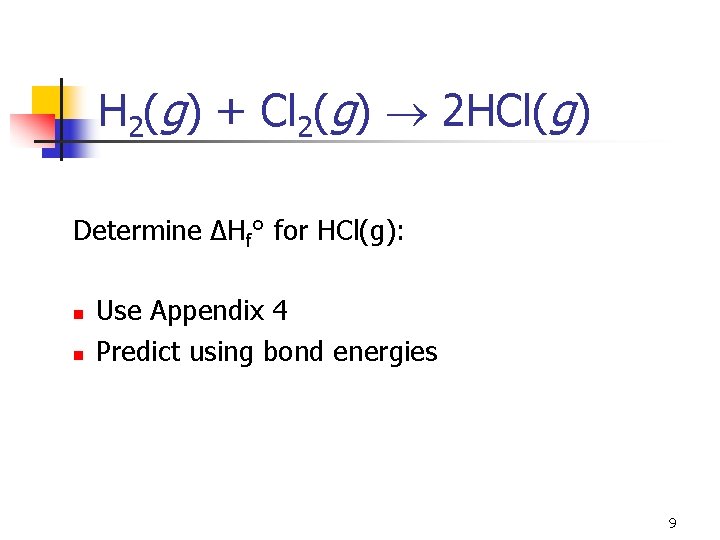
H 2(g) + Cl 2(g) 2 HCl(g) Determine ΔHf° for HCl(g): n n Use Appendix 4 Predict using bond energies 9

Lewis Structures • • Determine the total number of valence electrons in the molecule. Form at least one bond between atoms in the molecule (a pair of electrons is a bond) Arrange the rest of the electrons (in pairs) to satisfy the octet rule or duet rule (for hydrogen). Watch for exceptions. 10

Clicker Question Which of the following has a Lewis structure most like that of the carbonate ion? a) the nitrate ion b) the sulfate ion c) carbon dioxide d) ozone (O 3) e) nitrogen dioxide 11



















