As always n n OWL LonCapa assignments Lecture






- Slides: 11

As always… n n OWL Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

Office Hours 3014 Chemistry Annex n decoste@illinois. edu n 11 am-12 pm Mondays and Wednesdays (after lecture) n 10 -11 am Tuesdays and 1 -2 pm Thursdays n By appointment; open-door policy n 2

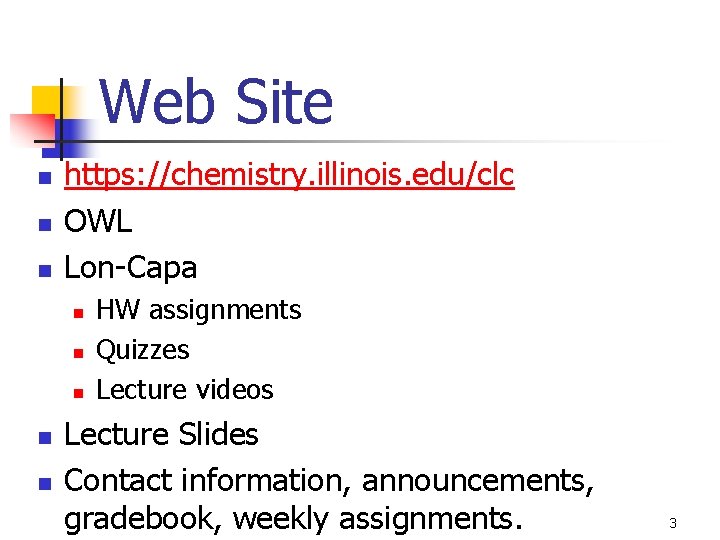
Web Site n n n https: //chemistry. illinois. edu/clc OWL Lon-Capa n n n HW assignments Quizzes Lecture videos Lecture Slides Contact information, announcements, gradebook, weekly assignments. 3

Three Big Topics n n n Stoichiometry: information on “what” happens; allows us to determine amounts of reactants and products. Thermodynamics: information on “why” reactions happen; allows us to make predictions about whether a reaction will occur. Kinetics: information on “how” reactions happen (mechanisms); relates to the speed of reactions. 4

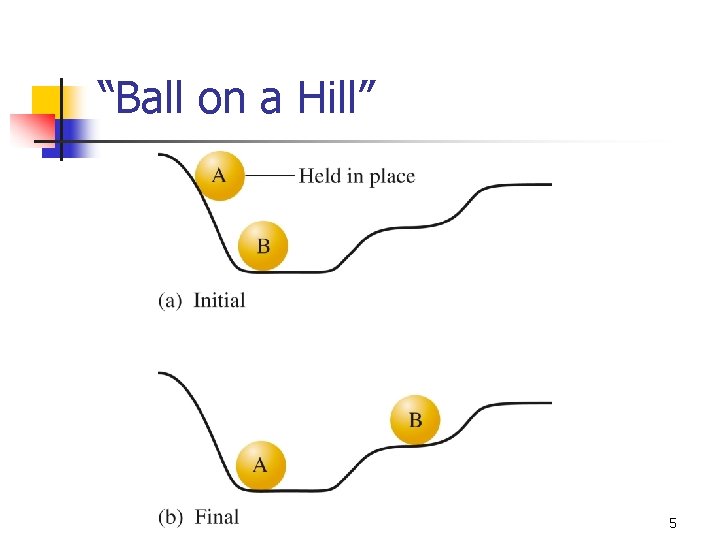
“Ball on a Hill” 5

Some Big Ideas n n Low energy low in potential energy more stable. If a system absorbs energy, the natural tendency is to release it. n n n That is, achieving low potential energy is “natural”. Ground state preferred over excited state. Energy is converted from one form to another but is NOT created nor destroyed. n n First law of thermodynamics. Energy is a state function (heat and work are not). 6

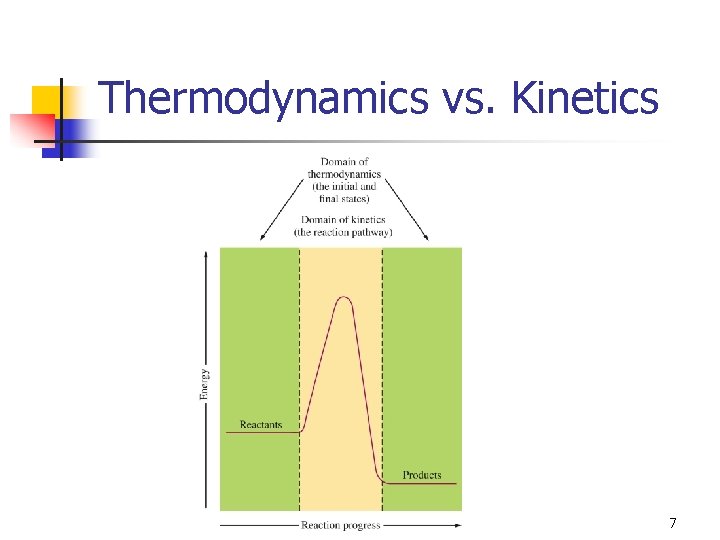
Thermodynamics vs. Kinetics 7

Questions To Consider n n How can we predict which reactions are spontaneous (and what do we mean by spontaneous)? How can we provide support (mathematical and qualitative) for observations? n n n Ice cream melts Coffee cools Why do systems reach equilibrium? That is, why doesn’t the ball go “all the way down the hill”? 8

Clicker Question The freezing of water is an ____ process. a) exothermic b) endothermic c) I do not know. 9

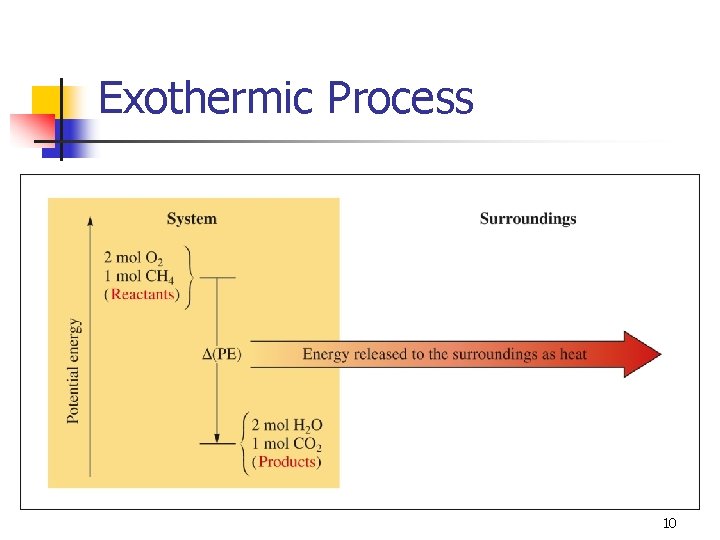
Exothermic Process 10

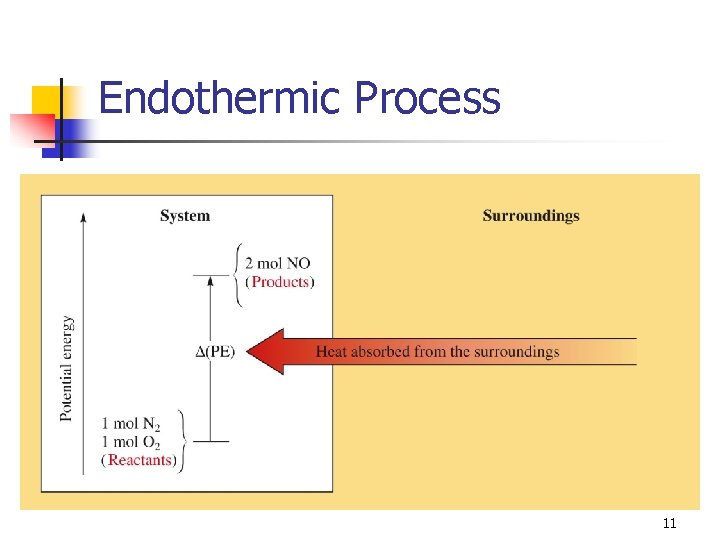
Endothermic Process 11



















