As always n n OWL LonCapa assignments Lecture




- Slides: 14

As always… n n OWL Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

Studying Gases Good way to study the process of science. n Distinguish between laws (what) and theories (why). n Make observations. n Use observations to develop theories. n Make more observations. n Continue. 2 n

Gases – Macroscopic Observations n Gases take up the volume of the container. n n n Gases exert pressure. n n 1 cup of helium? Compressible Basketballs, bike tires, etc. Explosions Density of a gas changes greatly with temperature. Gases mix well (diffusion). n We can smell ammonia, vinegar, etc. 3

Clicker Question You are holding two balloons filled with air – a large balloon and a small balloon. What can be said about the relative pressure of air in each balloon? a) The pressure in the larger balloon is greater because there are moles of gas. b) The pressure in the smaller balloon is greater because there is less space inside the balloon. c) The balloons have essentially the same pressure. 4

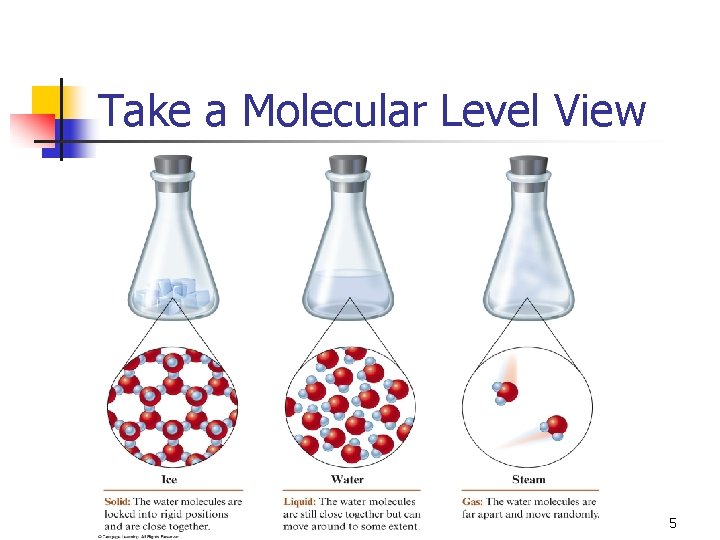
Take a Molecular Level View 5

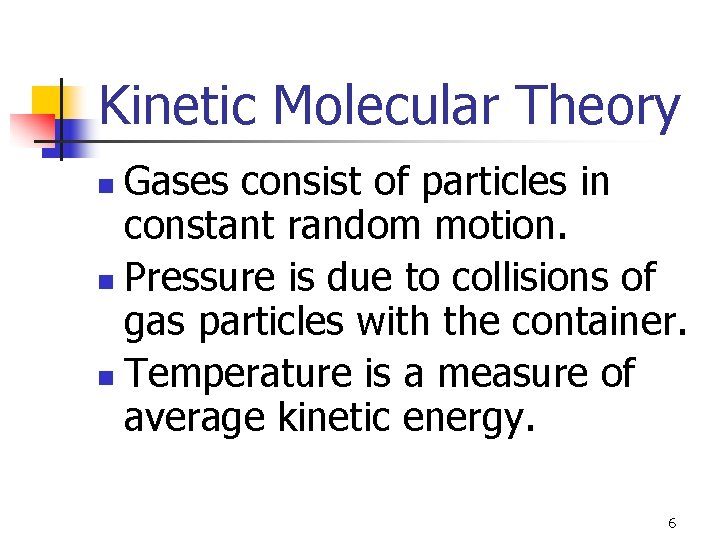
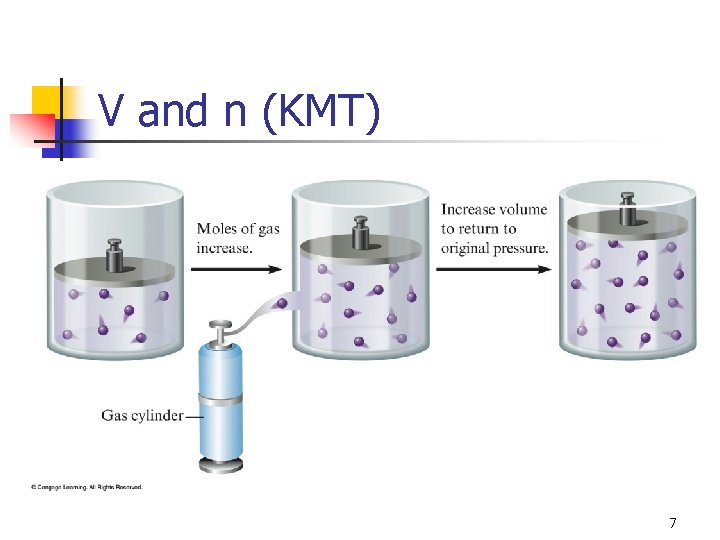
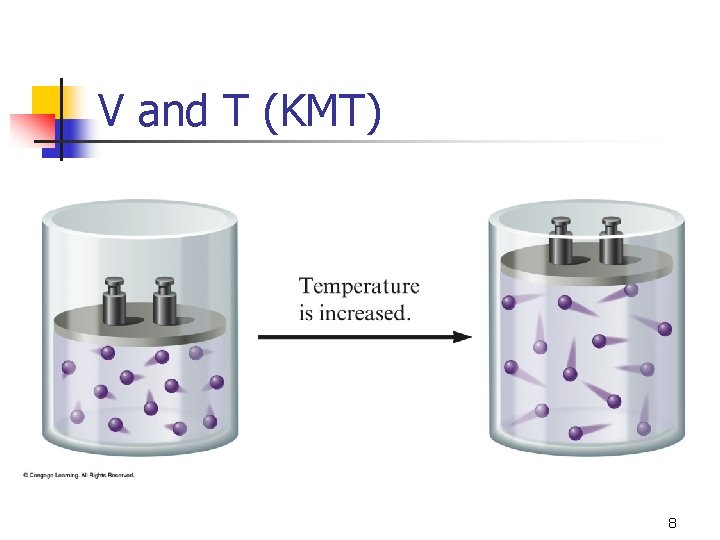
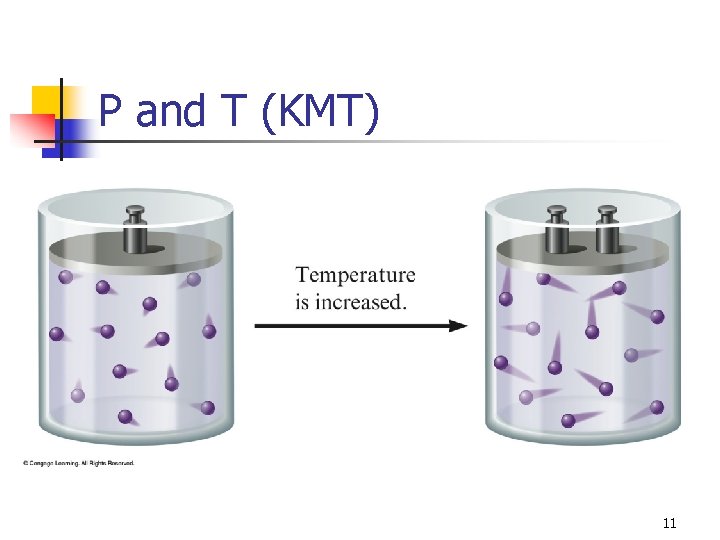
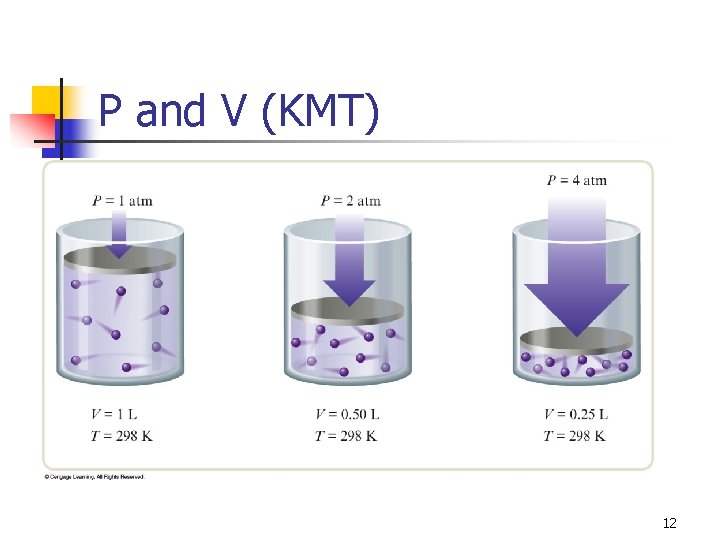
Kinetic Molecular Theory Gases consist of particles in constant random motion. n Pressure is due to collisions of gas particles with the container. n Temperature is a measure of average kinetic energy. n 6

V and n (KMT) 7

V and T (KMT) 8

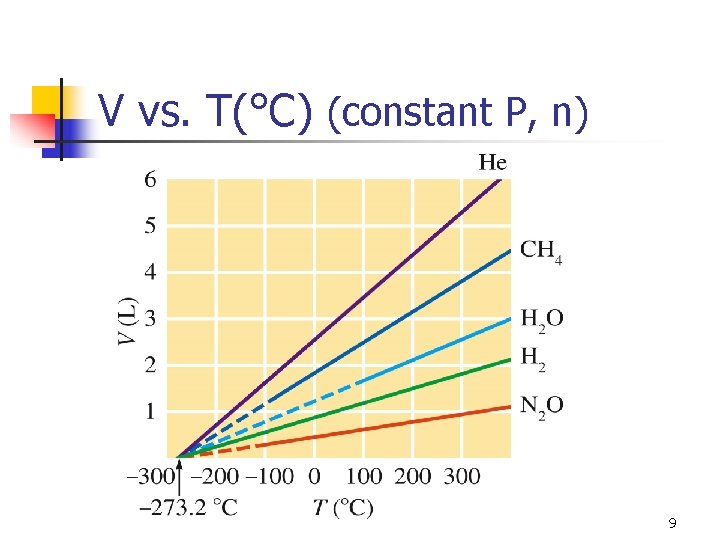
V vs. T(°C) (constant P, n) 9

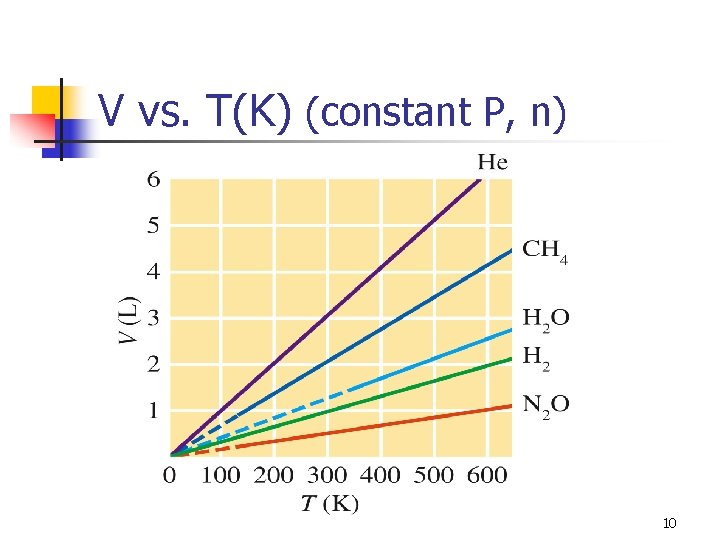
V vs. T(K) (constant P, n) 10

P and T (KMT) 11

P and V (KMT) 12

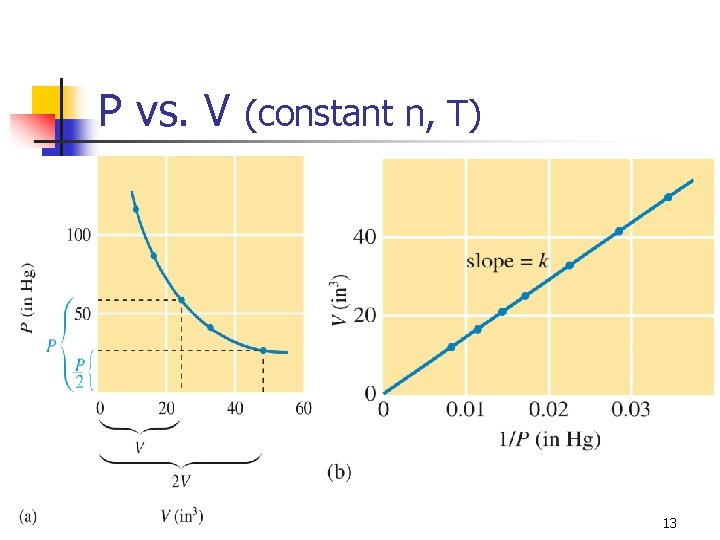
P vs. V (constant n, T) 13

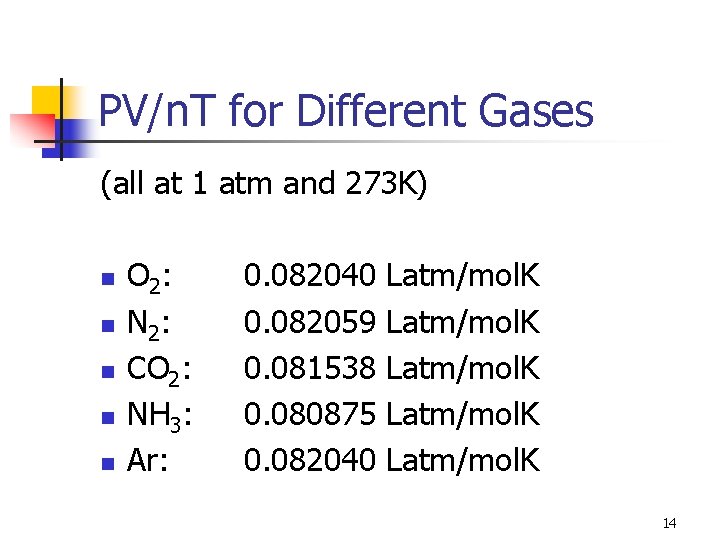
PV/n. T for Different Gases (all at 1 atm and 273 K) n n n O 2: N 2: CO 2: NH 3: Ar: 0. 082040 0. 082059 0. 081538 0. 080875 0. 082040 Latm/mol. K 14
 Https://owl.english.purdue.edu
Https://owl.english.purdue.edu Ohio university lon capa
Ohio university lon capa Loncapa
Loncapa Loncapa purdue
Loncapa purdue Capa assessment tool
Capa assessment tool Loncapa sfu
Loncapa sfu Hscn lewis structure
Hscn lewis structure Phy 2048c fsu
Phy 2048c fsu 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Walmart
Walmart Uts in text referencing
Uts in text referencing Challenge and change in society assignments
Challenge and change in society assignments Unit 2 technology systems
Unit 2 technology systems Sculpture assignments
Sculpture assignments They haven't finished their homework yet
They haven't finished their homework yet

























