An Anecdotal Study of Low Dose Naltrexone Skips



- Slides: 14

An Anecdotal Study of Low Dose Naltrexone Skip’s Pharmacy Boca Raton, Fl. Skip Lenz Pharm. D. F. A. S. C. P. with Research Assistants Shanna Chambliss Pharm. D. (Candidate) University of Florida Vinay Patel Pharm. D. (Candidate) University of Florida Bandar Saleh Pharm. D. (Candidate) P. B. A. School of Pharmacy Jeremy Thomas Pharm. D. (Candidate) University of Florida Hew Fong Pharm. D. (Candidate) University of Florida Felicia Fong Kong Pharm. D. (Candidate) University of Florida

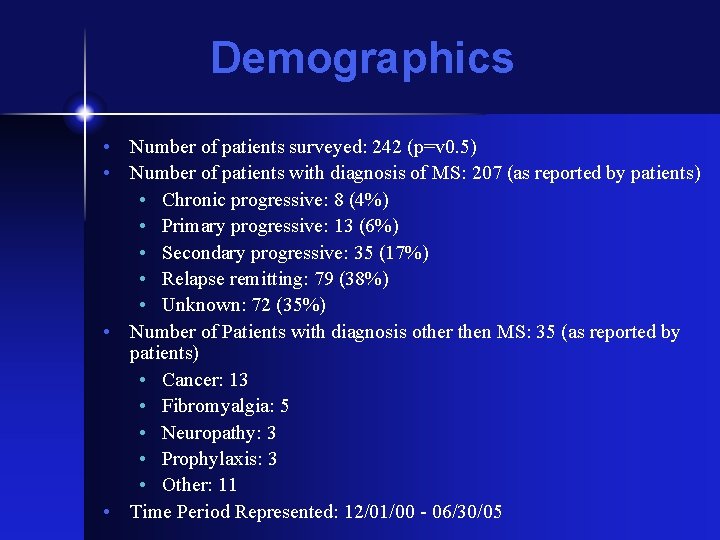
Demographics • Number of patients surveyed: 242 (p=v 0. 5) • Number of patients with diagnosis of MS: 207 (as reported by patients) • Chronic progressive: 8 (4%) • Primary progressive: 13 (6%) • Secondary progressive: 35 (17%) • Relapse remitting: 79 (38%) • Unknown: 72 (35%) • Number of Patients with diagnosis other then MS: 35 (as reported by patients) • Cancer: 13 • Fibromyalgia: 5 • Neuropathy: 3 • Prophylaxis: 3 • Other: 11 • Time Period Represented: 12/01/00 - 06/30/05

Questionnaire 1. What is the reason (your diagnosis) for taking LDN 2. How long have you had this condition/disease 3. (if the diagnosis is Multiple Sclerosis) Is the Condition type progressive or relapse/remitting? Or other 4. (if relapse remitting MS) When was your last exacerbation? 5. How would you rate your symptoms after starting LDN vs. before LDN a) Worsened b) No Change c) Improved 6. How long did it take for you to see a change in your symptoms since you started LDN?

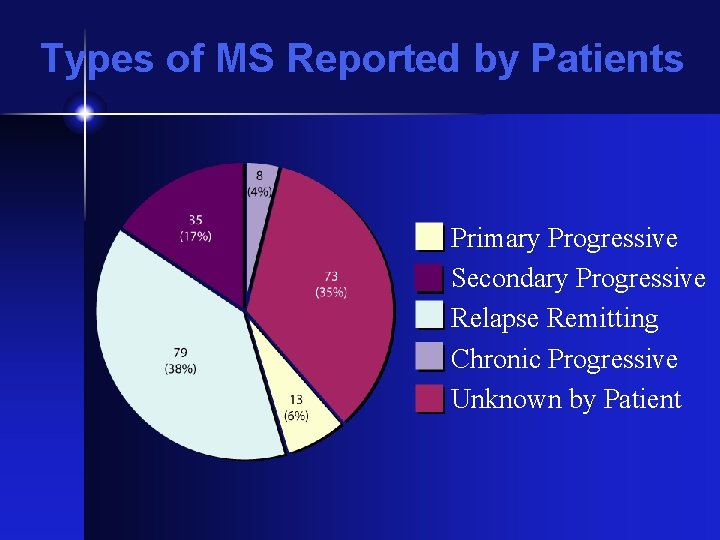
Types of MS Reported by Patients Primary Progressive Secondary Progressive Relapse Remitting Chronic Progressive Unknown by Patient

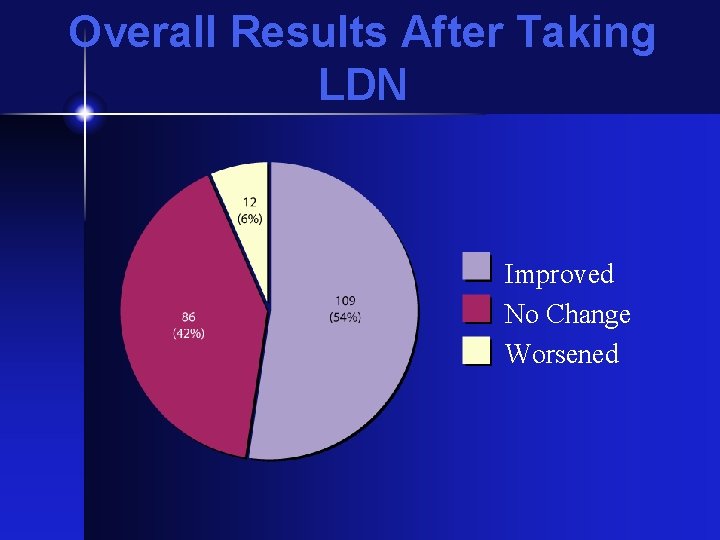
Overall Results After Taking LDN Improved No Change Worsened

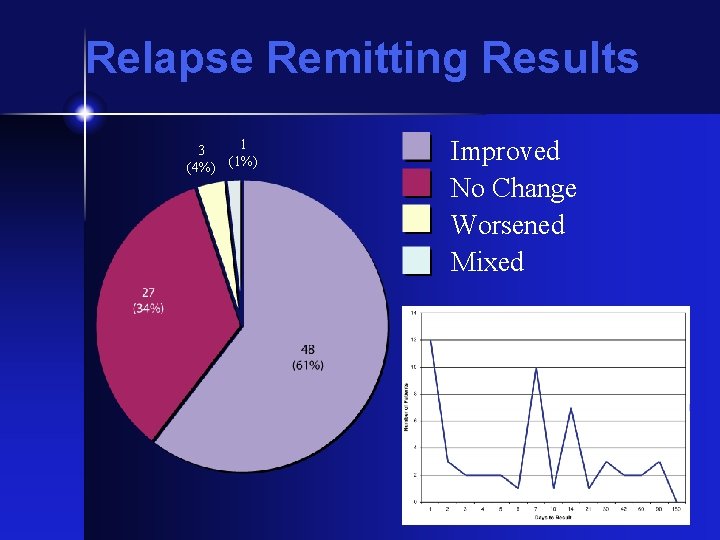
Relapse Remitting Results 3 (4%) 1 (1%) Improved No Change Worsened Mixed

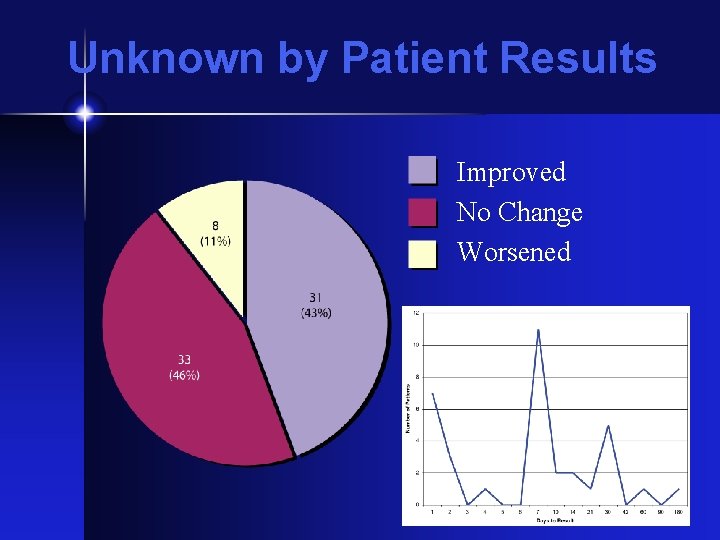
Unknown by Patient Results Improved No Change Worsened

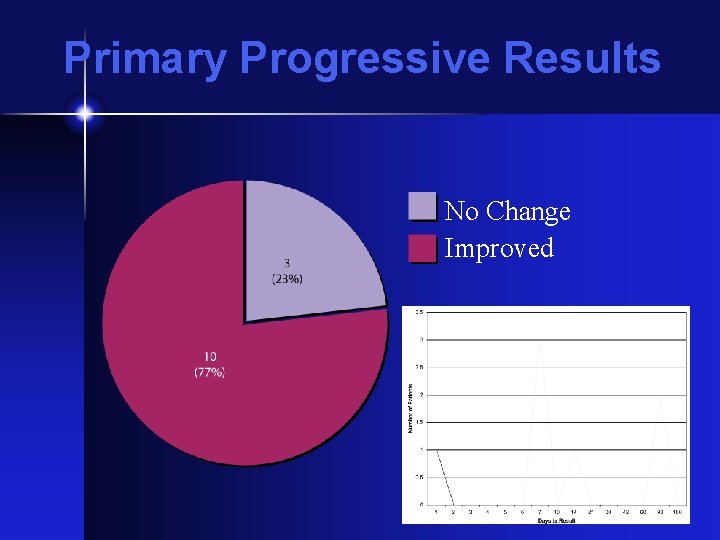
Primary Progressive Results No Change Improved

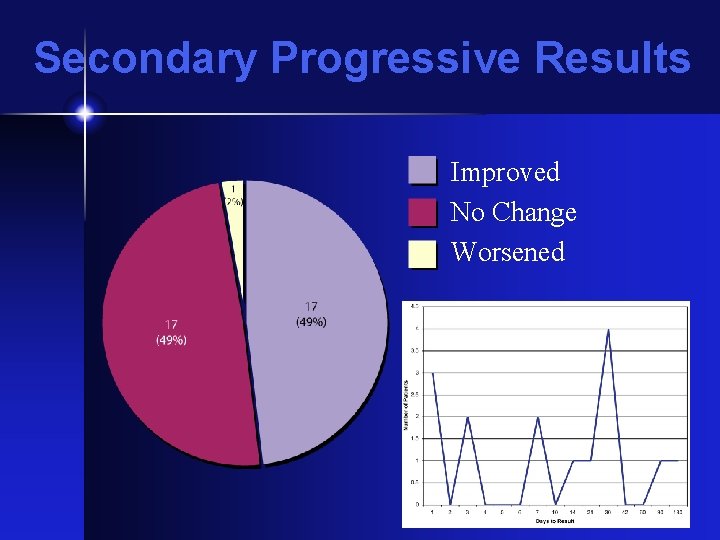
Secondary Progressive Results Improved No Change Worsened

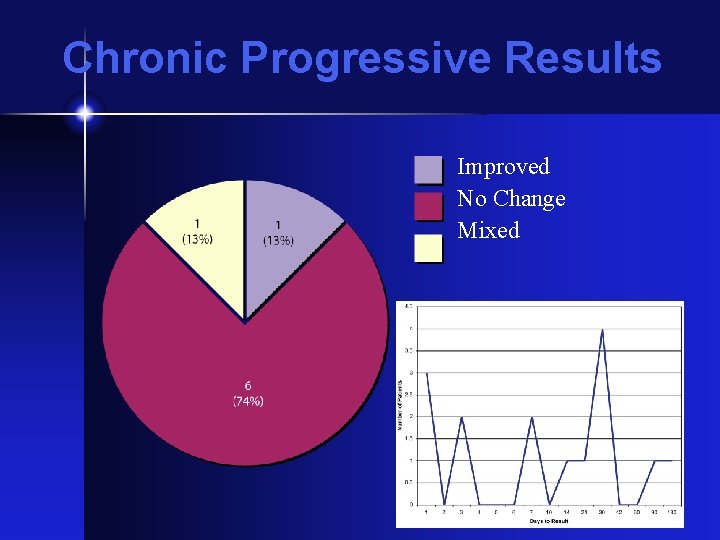
Chronic Progressive Results Improved No Change Mixed

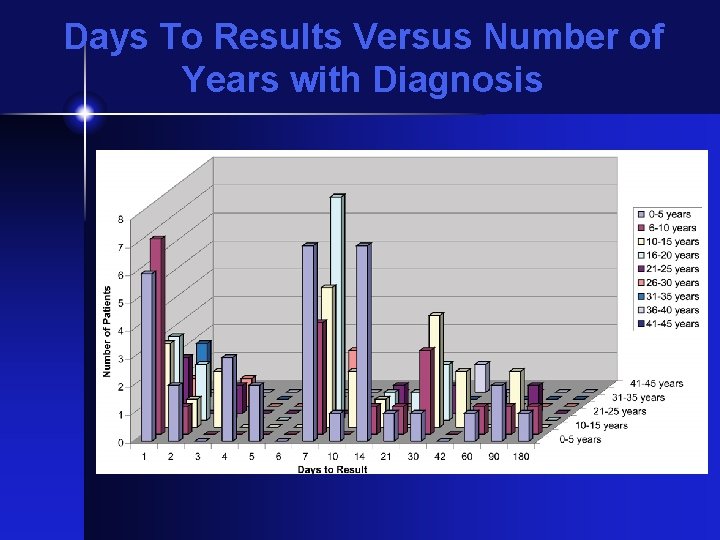
Days To Results Versus Number of Years with Diagnosis

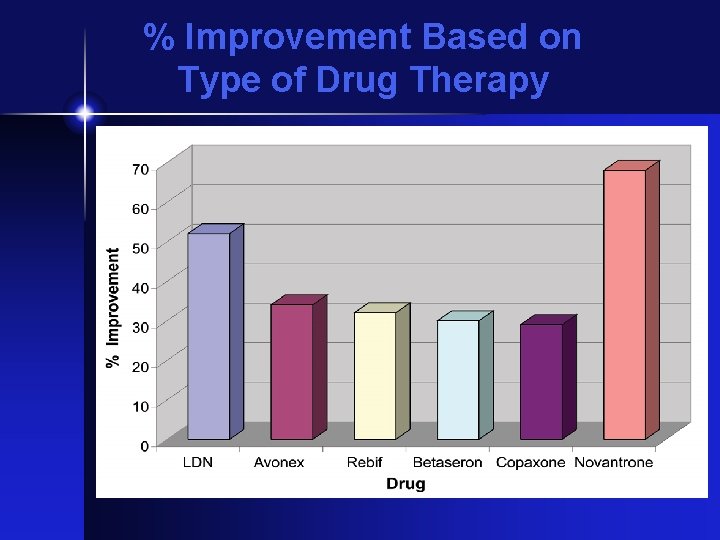
% Improvement Based on Type of Drug Therapy

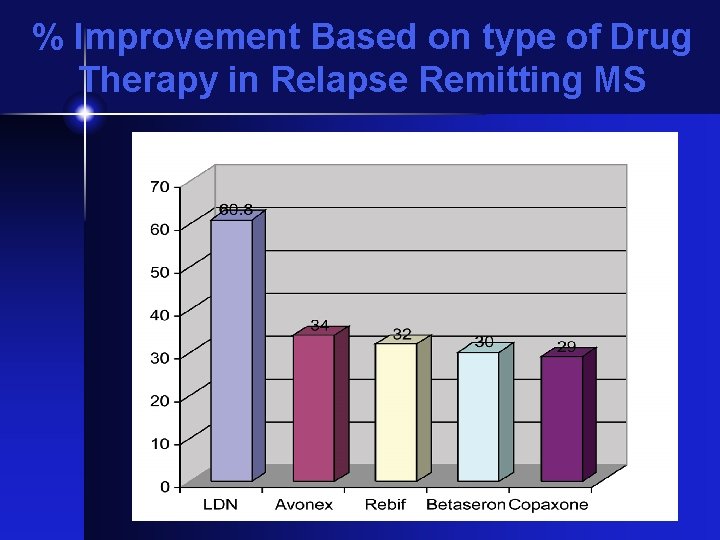
% Improvement Based on type of Drug Therapy in Relapse Remitting MS

Citations www. avonex. com www. rebif. com www. betaseron. com www. popaxone. com www. novantrone. com Goodin, D. S. "Disease Modifying Therapy in Multiple Sclerosis” Report of the Therapeutic and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology #58 Jan 2002 pg. 169 -178 Goodin, D. S. "The Use of Mitoxantrone (novantrone) for the treatment of Multiple Sclerosis" Report of the Therapeutic and Technology Assessment Subcommittee of the American Academy of Neurology #61 Nov. 2003 pg. 13321338
 Walgreen uti test
Walgreen uti test Breaking bad
Breaking bad Naltrexone black box warning
Naltrexone black box warning Naltrexone and autism
Naltrexone and autism Increase bp
Increase bp Tomo hd
Tomo hd Middle = low + (high - low) / 2
Middle = low + (high - low) / 2 Sociability continuum
Sociability continuum Low accuracy low precision
Low accuracy low precision Low voltage = low hazard
Low voltage = low hazard Running record observation samples
Running record observation samples Question lead in journalism
Question lead in journalism Nursing anecdotes examples
Nursing anecdotes examples Anecdotal example
Anecdotal example What is staccato lead
What is staccato lead

























