LDN Low Dose Naltrexone Special Thanks to Michael





















































- Slides: 67

LDN Low Dose Naltrexone Special Thanks to: Michael Gresh Pharm. D Candidate 2017 University of Connecticut

Katelyn Zachau, DO Board Certified Family Medicine Practicing Integrative Medicine & Primary Care Everwell Integrated Medicine, LLC Collaborative Natural Health Partners, LLC Gene Gresh, R. Ph. , FIACP, IFMCP Research and Compounding Specialist Fellow International Academy of Compounding Pharmacists Institute for Functional Medicine Certified Practitioner PIONEER HEALTH COMPOUNDING PHARMACY, LLC

Objectives S Review of Naltrexone S Introduction to Low Dose Naltrexone (LDN) S Mechanism of Action of LDN-cellular science S Studies on LDN S How to prescribe LDN S Clinical Experience with LDN

Naltrexone S Approved by FDA in 1984 S Opiate antagonist S Indicated to treat Opiate and Alcohol addiction S Dose: between 50 -300 mg per day http: //www. micromedexsolutions. com. ezproxy. lib. uconn. edu

Pharmicokinetics S Oral Absorption: S Almost complete up to 96% S Peak plasma levels occur within 1 hour S Metabolism: S Hepatic: significant first-pass resulting in 5 -40% bioavailability S Active metabolite: 6 -beta-naltrexol S Excretion: S Renal elimination: 53 -79% S Half Life S Naltrexone: 4 -6 hours S 6 -beta-naltrexol: 13 hours

Special warnings and precautions for use: S Adverse reaction with opioids – severe – ensure no opioid use!! S Confirm normal kidney and liver function S Some elderly patients on 300 mg naltrexone develop abnormal liver function tests

SAFETY S No evidence of toxicity in volunteers receiving 800 mg/day for seven days S Prolonged use at 50 mg is acceptable S duration of treatment is not limited

Low Dose Naltrexone aka “LDN” S

What is LDN and how does it work? S Dose: 1. 5 -4. 5 mg PO taken once nightly (or daily) S MOA: S Brief period of opioid blockade adaptive increase in endorphin and enkephalin production S Endorphin & Enkaphalin S Work on opioid receptors to produce analgesia S Increase in endorphins prolonged upregulation of important elements of the immune system S This paradoxical effect has not been seen with higher dosages (50 mg)

Proposed Indications for LDN S Autoimmune disorders S Malignancies/cancers S Cardiac diseases S Neurologic diseases S Dermatologic diseases S Ocular diseases S Ear, Nose, Sinus & Throat S Psychological disorders S Endocrine diseases S Pulmonary disease S Gastrointestinal diseases S Rheumatologic disorders S Hematologic/Blood marrow disorders S Urologic diseases S Infectious diseases S Vasculitis

LDN-History S In 1985, Bernard Bihari, MD, a physician with a clinical practice in New York City, discovered the effects of a much smaller dose of naltrexone (approximately 3 mg once a day) on the body's immune system. He found that this low dose, taken at bedtime, was able to enhance a patient's response to infection by HIV, the virus that causes AIDS. [Note: Subsequently, the optimal adult dosage of LDN has been found to be 4. 5 mg. ] S In the mid-1990's, Dr. Bihari found that patients in his practice with cancer (such as lymphoma or pancreatic cancer) could benefit, in some cases dramatically, from LDN. In addition, people who had an autoimmune disease (such as lupus) often showed prompt control of disease activity while taking LDN.

Mechanism(s) of Action Cellular Science

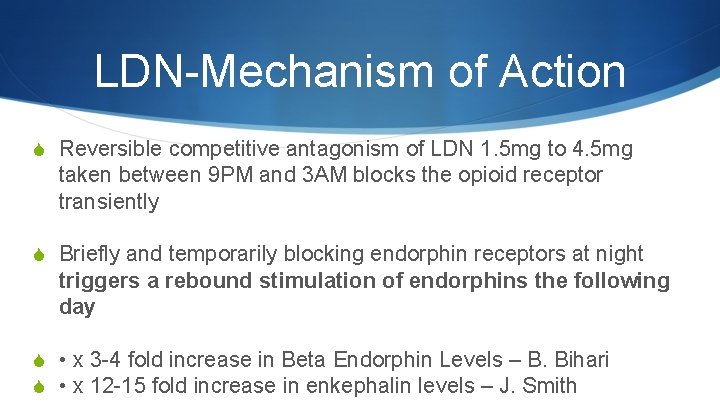
LDN-Mechanism of Action S Reversible competitive antagonism of LDN 1. 5 mg to 4. 5 mg taken between 9 PM and 3 AM blocks the opioid receptor transiently S Briefly and temporarily blocking endorphin receptors at night triggers a rebound stimulation of endorphins the following day S • x 3 -4 fold increase in Beta Endorphin Levels – B. Bihari S • x 12 -15 fold increase in enkephalin levels – J. Smith

LDN increases Endorphins & Enkephalins S S S S –Promote healing –Inhibit cell growth –Reduce inflammation –Positively augment the immune system –provide a sense of euphoria- “endorphin rush” –Provide a sense of well-being and satisfaction –Provide “natural” analgesia

ENDORPHINS S Our bodies natural “opioids” that provide pain relief, a sense of euphoria and a sense of completion. S Endorphin deficiency may play a role in many psychiatric conditions. . Depression, OCD, emotional instability or even self hurting behavior S Endorphins are quickly broken down by enzymes after binding to receptors which makes them not addictive---where as Opioid drugs resist this breakdown and extend the euphoria creating addictive behavior, dependence and a feedback decrease in ENDORPHINS

ENDORPHINS S Endorphins are naturally produced in response to pain and stress, but their production can also be triggered by various human activities. S Endorphins may be responsible for the "placebo effect, ” S response of endorphin-release prompted by a tricked hypothalamus, creating a sense of well-being after consuming a much-hyped sugar pill, or even after simply anticipating something pleasurable.

ENKEPHALINS S It is shown that lymphocytes have surface receptors for endorphins and enkephalins. Furthermore, endorphins and enkephalins can influence several immune functions such as antibody synthesis, lymphocyte proliferation, and natural killer cytotoxicity. It is thus possible that the receptors play a functional role Fed Proc. 1985 Jan; 44(1 Pt 1): 92 -4. Enkephalins and endorphins as modifiers of the immune system: present and future. Wybran J

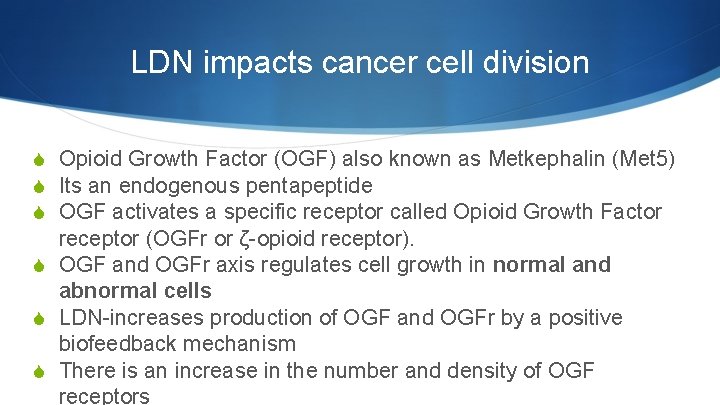
LDN impacts cancer cell division S Opioid Growth Factor (OGF) also known as Metkephalin (Met 5) S Its an endogenous pentapeptide S OGF activates a specific receptor called Opioid Growth Factor receptor (OGFr or ζ-opioid receptor). S OGF and OGFr axis regulates cell growth in normal and abnormal cells S LDN-increases production of OGF and OGFr by a positive biofeedback mechanism S There is an increase in the number and density of OGF receptors

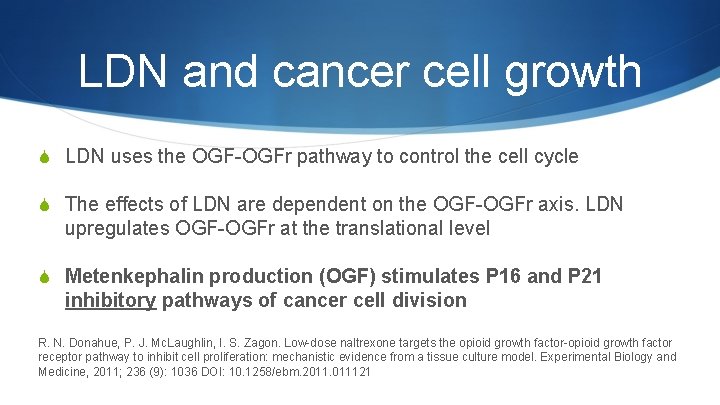
LDN and cancer cell growth S LDN uses the OGF-OGFr pathway to control the cell cycle S The effects of LDN are dependent on the OGF-OGFr axis. LDN upregulates OGF-OGFr at the translational level S Metenkephalin production (OGF) stimulates P 16 and P 21 inhibitory pathways of cancer cell division R. N. Donahue, P. J. Mc. Laughlin, I. S. Zagon. Low-dose naltrexone targets the opioid growth factor-opioid growth factor receptor pathway to inhibit cell proliferation: mechanistic evidence from a tissue culture model. Experimental Biology and Medicine, 2011; 236 (9): 1036 DOI: 10. 1258/ebm. 2011. 011121


LDN-another MOA S LDN has analgesic, anti-inflammatory, and neuroprotective properties that are NOT dependent on opioid receptor antagonism S Related to microglia activation in the nervous system

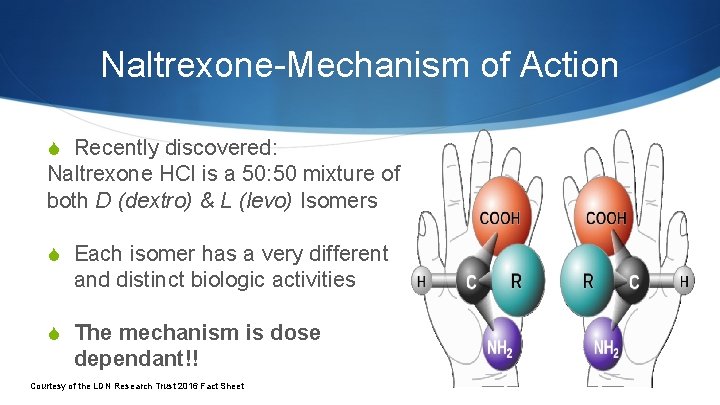
Naltrexone-Mechanism of Action S Recently discovered: Naltrexone HCl is a 50: 50 mixture of both D (dextro) & L (levo) Isomers S Each isomer has a very different and distinct biologic activities S The mechanism is dose dependant!! Courtesy of the LDN Research Trust 2016 Fact Sheet

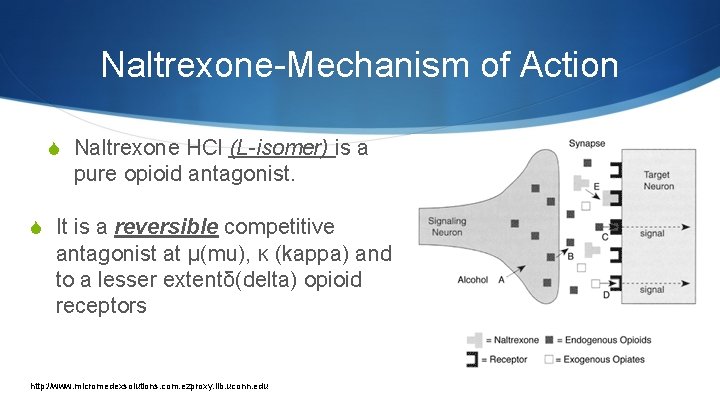
Naltrexone-Mechanism of Action S Naltrexone HCl (L-isomer) is a pure opioid antagonist. S It is a reversible competitive antagonist at μ(mu), ĸ (kappa) and to a lesser extentδ(delta) opioid receptors http: //www. micromedexsolutions. com. ezproxy. lib. uconn. edu

Naltrexone-Mechanism of Action S Naltrexone HCl (D-isomer) is an antagonist for certain immune cells such as Toll Like Receptors (i. e. TLR 4) S This antagonism results in decreased cytokines, TNF-a, ROS, NF-k. B S reduces inflammation S Potentially down regulating oncogenes SWang, X. , Zhang, Y. , Peng, Y. , Hutchinson, M. R. , Rice, K. C. , Yin, H. , and Watkins, L. R. (2016) Pharmacological characterization of the opioid inactive isomers NOTE: This immunomodulatory effect (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. British Journal of Pharmacology, 173: 856– 869. doi: 10. 1111/bph. 13394. 4 is NOT seen in doses of 50 -300 mg

LDN-TLR 4 S While blocking opioid receptors, LDN simultaneously blocks a non- opioid receptor called Toll-like receptor 4 (TLR 4). S TLR 4 is found on macrophages called microglia. S Microglia are central nervous system immune cells along with astrocytes represent 70 -80% of all CNS cells (aka Glia). S Triggers hit TLR 4 activation microglia production of inflammatory & excitatory factors S If microglia are chronically activated it can lead to neurotoxicity. Watkins, Hutchinson, Ledeboer, Milligan et al Brain Behav Immun 2007 Feb; 21(2): 131 -146 The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain Jarred Younger, Luke Parkitny, David Mc. Lain Clin Rheumatol. 2014; 33(4): 451– 459. Published online 2014 February 15. doi: 10. 1007/s 10067 -014 -2517 -2 PMCID: PMC 3962576

Activated Glia=opioid tolerance & hyperalgesia!!! S When activated – glia release a variety of substances (proinflammatory S S cytokines, chemokines, etc. ) resulting in inflammatory and excitatory factors that can cause sickness behaviors such as pain sensitivity, fatigue, cognitive disruption, sleep disorders, mood disorders, and general malaise These substances in turn increase the excitability of nearby neurons Its expression is up-regulated under neuroinflammatory conditions. Opioids cause glial cell activation by acting on the TLR 4 receptors leading to a cascade of pro- inflammatory cytokines –this may help explain Opioid Tolerance & Opioid induced hyperalgesia Opioid antagonists (Naloxone/Naltrexone) block TLR 4 signaling

Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids Linda R. Watkins a, ¤, 1, Mark R. Hutchinson a, Annemarie Ledeboer a, b, Julie Wieseler-Frank a, Erin D. Milligan a, Steven F. Maier Brain, Behavior, and Immunity 21 (2007) 131– 146 S S S “two recently recognized roles of glia (microglia and astrocytes) in: (a) creating and maintaining enhanced pain states such as neuropathic pain, and (b) compromising the efficacy of morphine and other opioids for pain control” While glia have little-to-no role in pain under basal conditions, pain is amplified when glia become activated, inducing the release of proinflammatory products, especially proinflammatory cytokines. glia become increasingly activated in response to repeated administration of opioids. Products of activated glia increase neuronal excitability via numerous mechanisms, including direct receptor-mediated actions, upregulation of excitatory amino acid receptor function, downregulation of GABA receptor function, and so on. These downstream effects of glial activation amplify pain, suppress acute opioid analgesia, contribute to the apparent loss of opioid analgesia upon repeated opioid administration (tolerance), and contribute to the development of opioid dependence.

STUDIES

“Low Dose Naltrexone as a Treatment for Active Crohn’s Disease” American Journal of Gastroenterology in 2007 Am J Gastroenterol. 2007 Apr; 102(4): 820 -8. Epub 2007 Jan 11. Low-dose naltrexone therapy improves active Crohn's disease. Smith JP 1, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS S

Trial Design S Dr. Jill Smith from Penn State led the open label trial. S Objective: safety and efficacy of LDN was tested in patients with active Crohn’s disease.

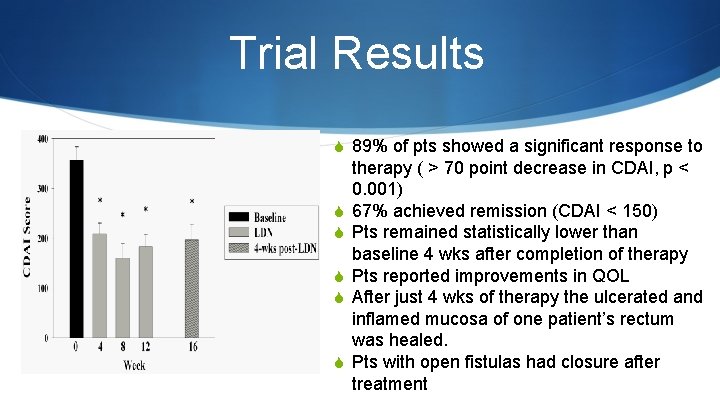
Trial Results S 89% of pts showed a significant response to S S S therapy ( > 70 point decrease in CDAI, p < 0. 001) 67% achieved remission (CDAI < 150) Pts remained statistically lower than baseline 4 wks after completion of therapy Pts reported improvements in QOL After just 4 wks of therapy the ulcerated and inflamed mucosa of one patient’s rectum was healed. Pts with open fistulas had closure after treatment

Conclusion S Dr. Smith concluded that LDN was effective and safe in the treatment of CD. S The only side effect seen was sleep disturbances (7 pts). S The compelling results allowed Dr. Smith to receive a grant from the NIH to run a phase 2 randomized double blind placebo controlled trial called “Therapy with the Opioid Antagonist Naltrexone Promotes Mucosal Healing in Active Crohn’s Disease: A Randomized Placebo-Controlled Trial”

“Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease” Digestive Diseases & Sciences in 2011 Dig Dis Sci. 2011 Jul; 56(7): 2088 -97. doi: 10. 1007/s 10620 -011 -1653 -7. Epub 2011 Mar 8. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn's disease: a randomized placebo-controlled trial. Smith JP 1, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, Mc. Govern CO, Zagon IS S

Trial Overview S 40 pts with CD were randomized to receive either 4. 5 mg naltrexone daily or placebo for 12 wks. S Primary outcome was a 70 point decline in CDAI. S Secondary outcome was mucosal healing based upon colonoscopy and appearance and histology.

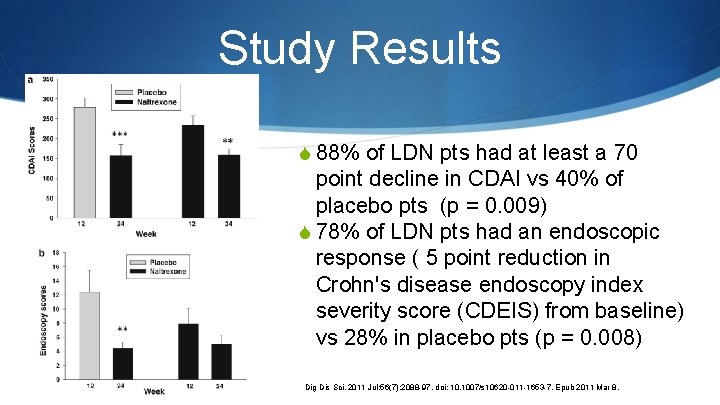
Study Results S 88% of LDN pts had at least a 70 point decline in CDAI vs 40% of placebo pts (p = 0. 009) S 78% of LDN pts had an endoscopic response ( 5 point reduction in Crohn's disease endoscopy index severity score (CDEIS) from baseline) vs 28% in placebo pts (p = 0. 008) Dig Dis Sci. 2011 Jul; 56(7): 2088 -97. doi: 10. 1007/s 10620 -011 -1653 -7. Epub 2011 Mar 8.

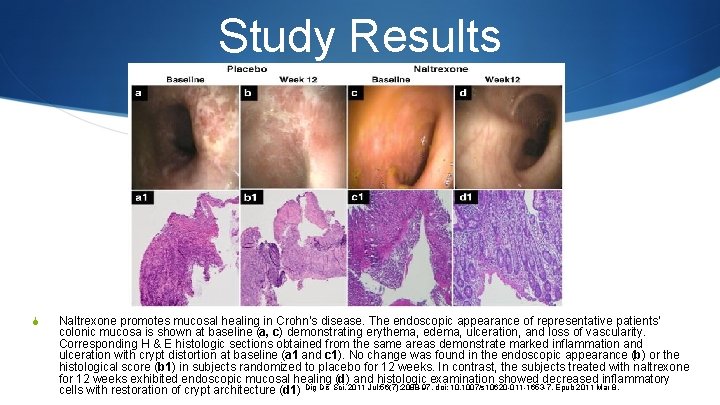
Study Results S Naltrexone promotes mucosal healing in Crohn’s disease. The endoscopic appearance of representative patients’ colonic mucosa is shown at baseline (a, c) demonstrating erythema, edema, ulceration, and loss of vascularity. Corresponding H & E histologic sections obtained from the same areas demonstrate marked inflammation and ulceration with crypt distortion at baseline (a 1 and c 1). No change was found in the endoscopic appearance (b) or the histological score (b 1) in subjects randomized to placebo for 12 weeks. In contrast, the subjects treated with naltrexone for 12 weeks exhibited endoscopic mucosal healing (d) and histologic examination showed decreased inflammatory cells with restoration of crypt architecture (d 1) Dig Dis Sci. 2011 Jul; 56(7): 2088 -97. doi: 10. 1007/s 10620 -011 -1653 -7. Epub 2011 Mar 8.

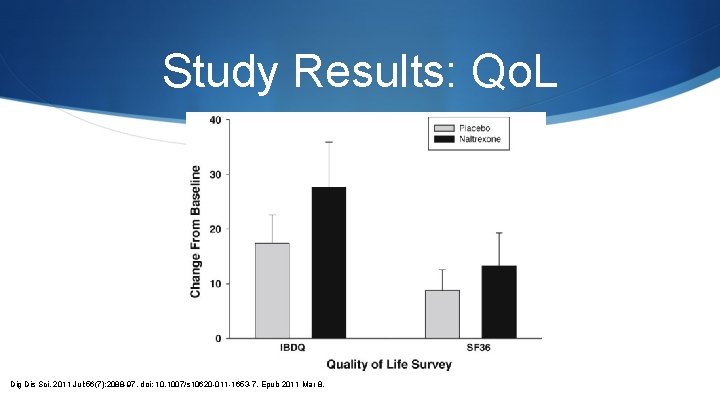
Study Results: Qo. L Dig Dis Sci. 2011 Jul; 56(7): 2088 -97. doi: 10. 1007/s 10620 -011 -1653 -7. Epub 2011 Mar 8.

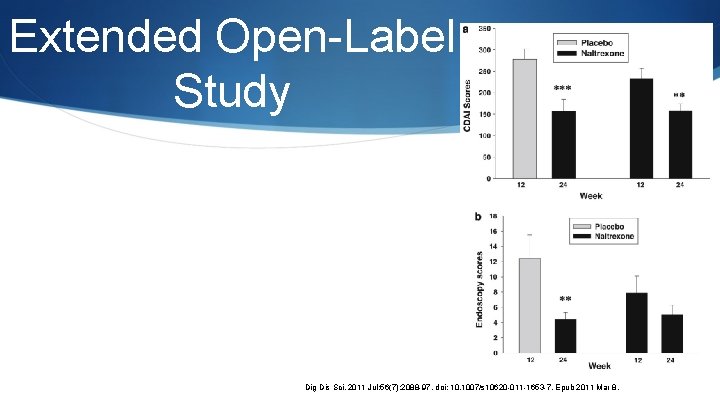
Extended Open-Label Study Dig Dis Sci. 2011 Jul; 56(7): 2088 -97. doi: 10. 1007/s 10620 -011 -1653 -7. Epub 2011 Mar 8.

LDN in Chronic Pain S

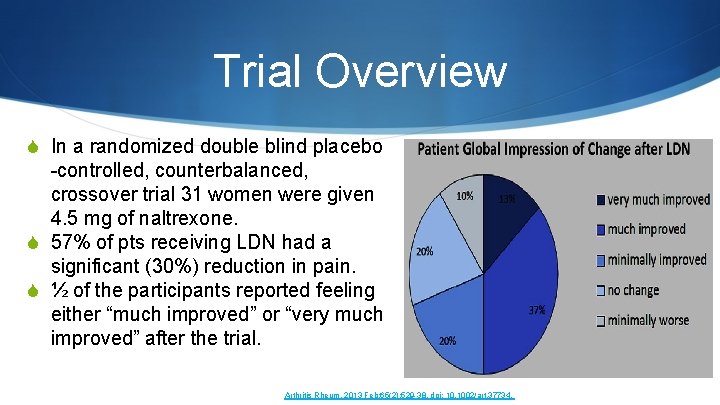
“Low Dose Naltrexone for the treatment of Fibromyalgia” Arthritis and Rheumatism in 2013 Arthritis Rheum. 2013 Feb; 65(2): 529 -38. doi: 10. 1002/art. 37734. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Younger J 1, Noor N, Mc. Cue R, Mackey S. S

Trial Overview S In a randomized double blind placebo -controlled, counterbalanced, crossover trial 31 women were given 4. 5 mg of naltrexone. S 57% of pts receiving LDN had a significant (30%) reduction in pain. S ½ of the participants reported feeling either “much improved” or “very much improved” after the trial. Arthritis Rheum. 2013 Feb; 65(2): 529 -38. doi: 10. 1002/art. 37734.

Authors Conclusion S The preliminary evidence continues to show that low-dose naltrexone has a specific and clinically beneficial impact on fibromyalgia pain. The medication is widely available, inexpensive, safe, and well-tolerated.

“The use of low-dose Naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain” Clinical Rheumatology in 2014 Clin Rheumatol. 2014; 33(4): 451– 459. Published online 2014 Feb 15. doi: 10. 1007/s 10067 -014 -2517 -2 PMCID: PMC 3962576 The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain Jarred Younger, Luke Parkitny, and David Mc. Lain S

Authors Conclusion S “The totality of the basic and clinical research to date suggests that LDN is a promising treatment approach for chronic pain conditions thought to involve inflammatory processes. . ” S “LDN may emerge as the first of many glial cell modulators that could be used to treat chronic conditions” S “As conventional anti-inflammatories have poor blood brainbarrier permeability, we expect centrally active immune modulators to be an area of interest in the future. ”

“Treatment of Complex Regional Pain Syndrome (CRPS) Using Low Dose Naltrexone (LDN)” Journal of Neuroimmune Pharmacology in 2013 Journal of Neuroimmune Pharmacology June 2013, Volume 8, Issue 3, pp 470 -476 First online: 02 April 2013 Treatment of Complex Regional Pain Syndrome (CRPS) Using Low Dose Naltrexone (LDN) Pradeep Chopra , Mark S. Cooper 10. 1007/s 11481 -013 -9451 -y S

Study Abstract S Complex Regional Pain Syndrome (CRPS) is a neuropathic pain syndrome, which involves glial activation and central sensitization in the central nervous system. Here, we describe positive outcomes of two CRPS patients, after they were treated with lowdose naltrexone (a glial attenuator), in combination with other CRPS therapies. Prominent CRPS symptoms remitted in these two patients, including dystonic spasms and fixed dystonia (respectively), following treatment with low-dose naltrexone (LDN). LDN, which is known to antagonize the Toll-like Receptor 4 pathway and attenuate activated microglia, was utilized in these patients after conventional CRPS pharmacotherapy failed to suppress their recalcitrant CRPS symptoms.

Authors Conclusion S Our use of LDN treatment for CRPS patients was motivated by a presumed neuroinflammatory etiology for long-standing CRPS symptoms. The remission of pain and dystonic spasms in Case 1, as well a remission of all CRPS symptoms (including fixed dystonia) in Case 2, provide evidence that a multi-modal interventional approach, which includes low-dose naltrexone (a known glial attenuator), should be considered as a treatment option for the treatment of CRPS patients, particularly those patients with dystonic movement disorders

“Low dose naltrexone in the treatment of dissociative symptoms” Nervenarzt. 2015 Mar; 86(3): 346 -51. doi: 10. 1007/s 00115 -014 -4015 -9. [Low dose naltrexone in the treatment of dissociative symptoms]. [Article in German] Pape W 1, Wöller W. S

Study Results S The low dose treatment with naltrexone proved to be effective whereby 11 out of 15 patients reported immediate positive effects and 7 described a lasting helpful effect. The majority of patients who felt positive effects reported a clearer perception of both their surroundings and their inner life. Assessment of reality and dealing with it improved as did the perception of their own body and affects as well as self-regulation. The treatment was very low in side effects.

Authors Conclusion S Treatment with low-dose naltrexone may be a helpful element in the treatment of patients with complex posttraumatic stress disorder. However, it has to be realized that the decrease of dissociation may lead patients to a not yet resolvable challenge, in as much as dissociation had previously been a necessary mechanism of self-protection

LDN-Female Reproductive Disorders S

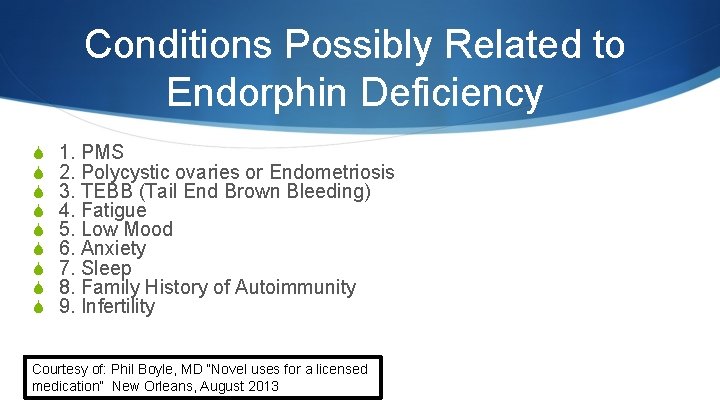
Conditions Possibly Related to Endorphin Deficiency S S S S S 1. PMS 2. Polycystic ovaries or Endometriosis 3. TEBB (Tail End Brown Bleeding) 4. Fatigue 5. Low Mood 6. Anxiety 7. Sleep 8. Family History of Autoimmunity 9. Infertility Courtesy of: Phil Boyle, MD “Novel uses for a licensed medication” New Orleans, August 2013

LDN-PMS S Phil Boyle, MD “Clinical experience in treating PMS is 80% response” S • Many say – I have my life back – I am me again!! The International Institute for Restorative Reproductive Medicine www. iirrm. org – “We intend to do a clinical trial with LDN 3 -4. 5 mg nightly” Courtesy of: Phil Boyle, MD “Novel uses for a licensed medication” New Orleans, August 2013

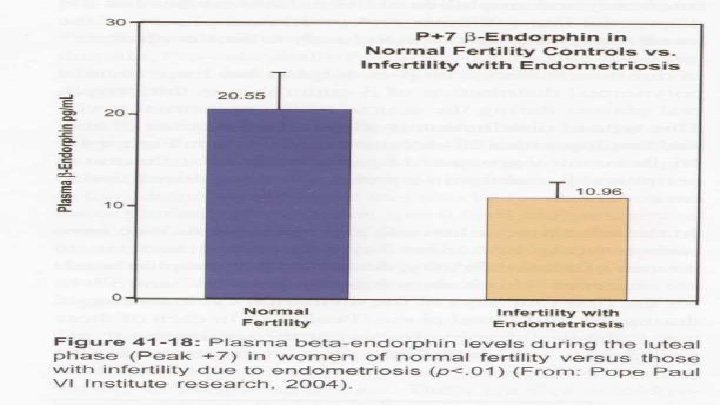
Current concepts of beta-endorphin physiology in female reproductive dysfunction Elevated or high levels of beta-endorphin have been associated with: S Exercise associated amenorrhea, stress-associated amenorrhea, and polycystic ovarian syndrome. …. . (consider: High Dose Naltrexone 25 mg BID) Depressed or low levels of beta-endorphin have been associated with: S PMS and menopause, (Endometriosis – Hilgers) ……(LDN 34. 5 mg nightly) Galway, Ireland Fertility and Sterility 1990. Seifer DB et al, Yale University School of Medicine http: //www. ncbi. nlm. nih. gov/pubmed/2226908


LDN-PRESCRIBING INFO S Start with 1. 5 mg QD HS x 2 weeks S Side effects (almost never happens at this dose) S Beneficial effect- continue at current dose S No effect. . Increase dose to 3 mg QD HS x 2 weeks S 3 mg QD x 2 weeks S Side effects- decrease dose for 7 days S Beneficial effect- continue at current dose S No effect. . Increase dose to 4. 5 mg QD HS x 2 weeks S Usual Maintenance Dose: 4. 5 mg HS S Side effects- decrease dose for 7 days

LDN-Compounding S • Needs to be specially compounded as a Rapid Release* preparation S (just means NOT a SLOW release formulation) S – DO NOT use lactose or calcium carbonate as a filler S – Preferably microcrystalline filler (avicel)

CLINICAL CONSIDERATIONS

LDN-possible Side Effects S Vivid Dreams S Sleep Disturbance S Nausea (generally will last for about 2 weeks) S Headache S Dry Mouth (over 95% acceptable)

LDN- Drug Interactions S Opioids (including Tramadol) S • Safe to combine with steroids S • Suggest to discontinue LDN 2 days before surgery and resume after stopping pain relief

LDN-Cautions S LDN is an opioid antagonist- may need to wean patients currently on long term opioids over 10 -14 days before starting LDN S Hashimoto’s Thyroiditis patients on Thyroid replacement may need to start at 1. 5 mg HS and monitor for a decrease in Thyroid dose as the LDN may lead to a prompt decrease in the condition S Patients who have had organ transplants and are taking immunosuppressive medications are cautioned against taking

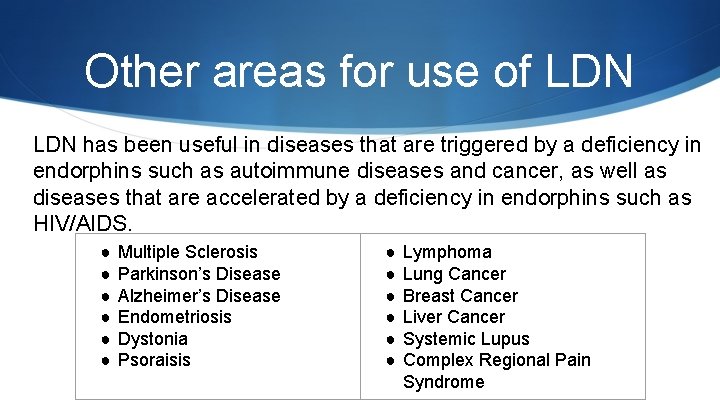
Other areas for use of LDN has been useful in diseases that are triggered by a deficiency in endorphins such as autoimmune diseases and cancer, as well as diseases that are accelerated by a deficiency in endorphins such as HIV/AIDS. ● ● ● Multiple Sclerosis Parkinson’s Disease Alzheimer’s Disease Endometriosis Dystonia Psoraisis ● ● ● Lymphoma Lung Cancer Breast Cancer Liver Cancer Systemic Lupus Complex Regional Pain Syndrome

LDN Therapy? ? ? S S Cancer Autoimmune Diseases HIV/AIDS CNS Disorders S Anecdotal reports continue to be received concerning beneficial effects of LDN on the course of Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS—Lou Gehrig’s disease), and primary lateral sclerosis. Dr. Jaquelyn Mc. Candless has found a very positive effect of LDN, in appropriately reduced dosage and applied as a transdermal cream, in children with autism.

Low-dose naltrexone for disease prevention and quality of life Norman Brown a, *, Jaak Panksepp b Medical Hypotheses June 2008 S Accumulating evidence suggests that LDN can promote health supporting immune-modulation which may reduce various oncogenic and inflammatory autoimmune processes. S Since LDN can upregulate endogenous opioid activity, it may also have a role in promoting stress resilience, exercise, social bonding, and emotional well-being, as well as amelioration of psychiatric problems such a autism and depression. S It is proposed that LDN can be used effectively as a buffer for a large variety of bodily and mental ailments through its ability to beneficially modulate both the immune system and the brain neurochemistries that regulate positive affect.

Low-Dose Naltrexone in Diseases’ Treatment: Global Review Research Inventy: International Journal of Engineering And Science Vol. 6, Issue 2 (February 2016), PP -01 -04 Issn (e): 2278 -4721, Issn (p): 2319 -6483, www. researchinventy. com Authors Conclusion: In reviewing the published literature on LDN we conclude that 3 to 4. 5 mg per day in humans is effective for idiopathic diseases with alterations in immune system, as well as those ones with inflammatory and tumor characteristics.


Questions?
 Macrobid price walgreens
Macrobid price walgreens Ldn and diabetes
Ldn and diabetes Dr. leonard weinstock
Dr. leonard weinstock Breaking bad
Breaking bad Naltrexone black box warning
Naltrexone black box warning Naltrexone and autism
Naltrexone and autism Michael w smith give thanks to the lord
Michael w smith give thanks to the lord Acknowledgement special thanks to
Acknowledgement special thanks to Dobutamine low dose
Dobutamine low dose Hologic's low dose 3d mammography
Hologic's low dose 3d mammography Mid low high
Mid low high What is communication style bias?
What is communication style bias? Low accuracy low precision
Low accuracy low precision Low voltage = low hazard
Low voltage = low hazard Alleluia alleluia give thanks to the risen lord lyrics
Alleluia alleluia give thanks to the risen lord lyrics American satan cda
American satan cda Thank you your listening
Thank you your listening Thanks for listen
Thanks for listen Gustation and olfaction
Gustation and olfaction Thanks for listening
Thanks for listening How to reply thanks
How to reply thanks Hypernates
Hypernates Give thanks to the lord our god and king
Give thanks to the lord our god and king Thanks for your attention doctor
Thanks for your attention doctor Thanks to god for my redeemer
Thanks to god for my redeemer Oh give thanks to the lord for he is good
Oh give thanks to the lord for he is good Thanks for your attention
Thanks for your attention 謝千萬聲
謝千萬聲 Thanks for your listening.
Thanks for your listening. Thanks for teamwork
Thanks for teamwork Kwakiutl prayer of thanks
Kwakiutl prayer of thanks Thank you a lot for your last letter
Thank you a lot for your last letter Vote of thanks to teachers
Vote of thanks to teachers Is the gulf stream warm or cold
Is the gulf stream warm or cold Kwakiutl prayer of thanks
Kwakiutl prayer of thanks Dear ben thank you for your letter
Dear ben thank you for your letter Gartner erp matrix
Gartner erp matrix First of all thanks to allah
First of all thanks to allah My dear student
My dear student Welcome thanks for joining us
Welcome thanks for joining us Thanks for bringing us together
Thanks for bringing us together Thanks for being here
Thanks for being here Thanks to all our sponsors
Thanks to all our sponsors Thank you for your kind attention.
Thank you for your kind attention. Thanks for solving the problem
Thanks for solving the problem Oh give thanks to the lord for he is good
Oh give thanks to the lord for he is good In everything give thanks to the lord
In everything give thanks to the lord How to reply thanks
How to reply thanks Conclusion to presentation
Conclusion to presentation Barry gane
Barry gane Complete each space with one word
Complete each space with one word Vote of thanks
Vote of thanks Cx hy oz
Cx hy oz Thanks for your attention
Thanks for your attention Thanks
Thanks Thanks for the rescue
Thanks for the rescue Thanks for your attention
Thanks for your attention Thanks for listening
Thanks for listening Thanks bri
Thanks bri Goodbye mission control thanks for trying
Goodbye mission control thanks for trying Thanks 2021
Thanks 2021 Thanks paul
Thanks paul Praise god dinda
Praise god dinda Thanks for your attention
Thanks for your attention Thanks
Thanks Thanks to provision
Thanks to provision Thanks for the reminder.
Thanks for the reminder. Alleluia alleluia give thanks to the risen lord
Alleluia alleluia give thanks to the risen lord