Low Dose Naltrexone Mechanisms of Action and Clinical


































- Slides: 58

Low Dose Naltrexone Mechanisms of Action and Clinical Applications Leonard Weinstock, MD Associate Professor of Clinical Medicine Washington University in St. Louis President, Specialists in Gastroenterology


Naltrexone n Anti-opioid n Approved by the FDA in 1985 to treat opiate dependence (Revia®, Depade® and extended-release Vivitrol® n Dose of 50 mg– 100 mg daily for opiate dependence

Anti-opioids n Mu opioid receptor antagonists: n Naltrexone n Methyl-naltrexone (Relistor) n Alvimopan (Enterg) n Kappa opioid receptor agonists: n Fedotozine; Asimadoline and ADL 10 - 0101 n Combined Mu antagonist/delta agonist n Eluxadoline (Mu. Delta)

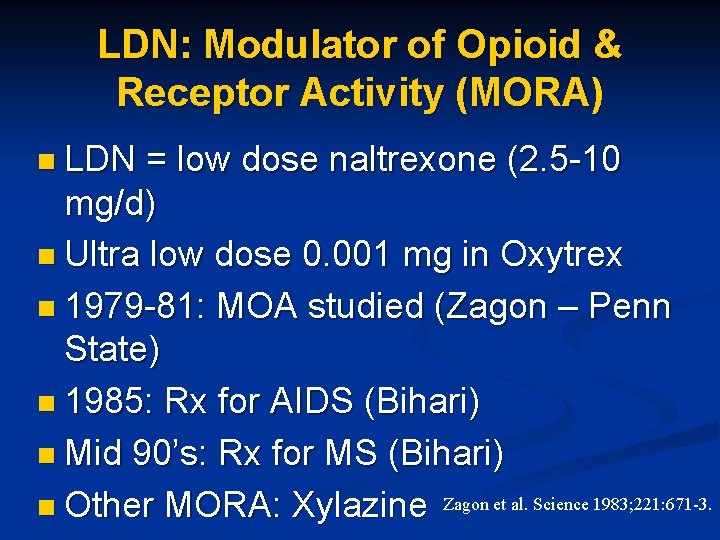
LDN: Modulator of Opioid & Receptor Activity (MORA) n LDN = low dose naltrexone (2. 5 -10 mg/d) n Ultra low dose 0. 001 mg in Oxytrex n 1979 -81: MOA studied (Zagon – Penn State) n 1985: Rx for AIDS (Bihari) n Mid 90’s: Rx for MS (Bihari) n Other MORA: Xylazine Zagon et al. Science 1983; 221: 671 -3.

Case 1 n 40 y. o. WF with Crohn’s disease – s/p total colectomy, recurrence in ileum 4 yrs later n Failing infliximab: diarrhea and fatigue

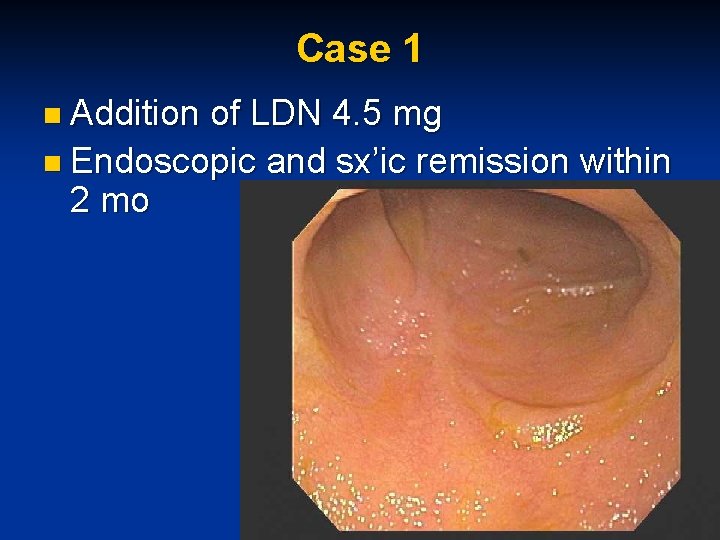
Case 1 n Addition of LDN 4. 5 mg n Endoscopic and sx’ic remission within 2 mo

Case 2 n 60 y. o. WF with 3 yr Hx RLS, constipation and halitosis n LBT: methane excretor n Rifaximin 550 mg TID 14 days n Naltrexone 2. 5 q. HS n Remission 6 years

St. Louis experience n 264 patients in 5 years (through 10/13) n 5 disorders n SIBO – second phase Rx – 108 n IBS (with negative LBT) – 83 n CIC – 27 n Crohn’s disease – 34 n Ulcerative colitis – 12 Weinstock. EMR search: 6/08 – 9/13.

LDN Treatment for Diseases Published Studies n Crohn’s Diseases n Irritable bowel n Multiple Sclerosis n Fibromyalgia n Complex regional pain syndrome n Cancer Submitted Study n Ulcerative colitis Patient-Reported n Ankylosing Spondylitis n Epstein-Barr Syndrome n Hepatitis C n Lung Cancer n Rheumatoid Arthritis n Lupus Erythematosus n Parkinson's Disease n Ulcerative Colitis

Placebo effect and Perceptions “Therapeutic reports with controls tend to have no enthusiasm and reports with enthusiasm tend to have no controls”

Endorphins n Endorphins produced in most cells n Regulate cell growth including immune cells n Disorders of the immune system can occur with unusually low levels of these endorphins n Met-Enkephalin is the most influential endorphin

Endogenous opioids & receptors n Peptides: B-endorphin, enkephalins, endomorphin, dynorphin n Receptors n CNS and PNS n GI tract n Myenteric plexus n Mucosal plexus n Endocrine cells of intestinal mucosa n Lymphocytes

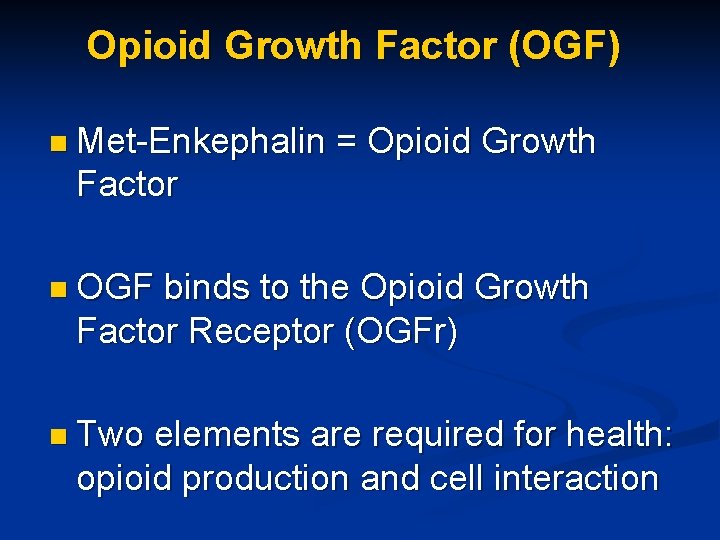
Opioid Growth Factor (OGF) n Met-Enkephalin = Opioid Growth Factor n OGF binds to the Opioid Growth Factor Receptor (OGFr) n Two elements are required for health: opioid production and cell interaction

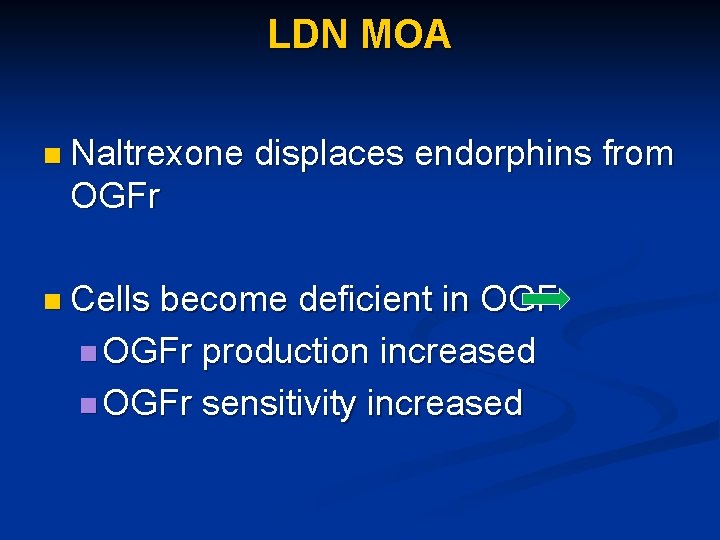
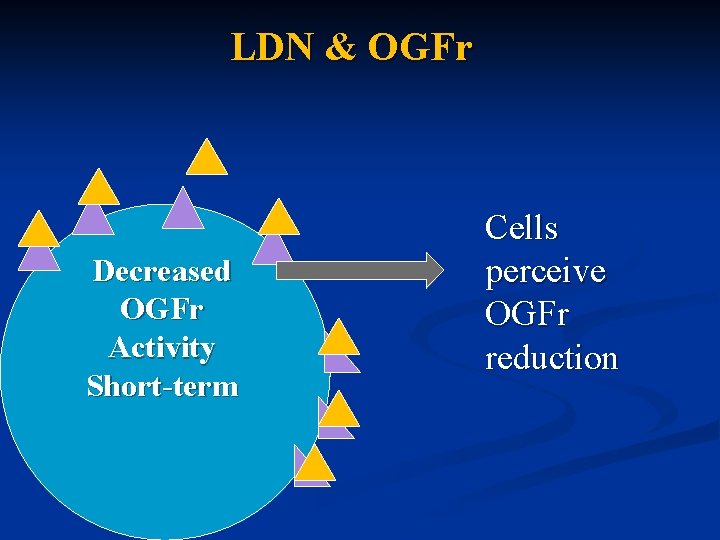
LDN MOA Naltrexone displaces endorphins bound to OGF receptors for 4 – 6 hours n Affected cells become deficient in OGF and results in: n n Receptor production and sensitivity is increased to capture more OGF n Production of OGF is increased to compensate for the perceived shortage

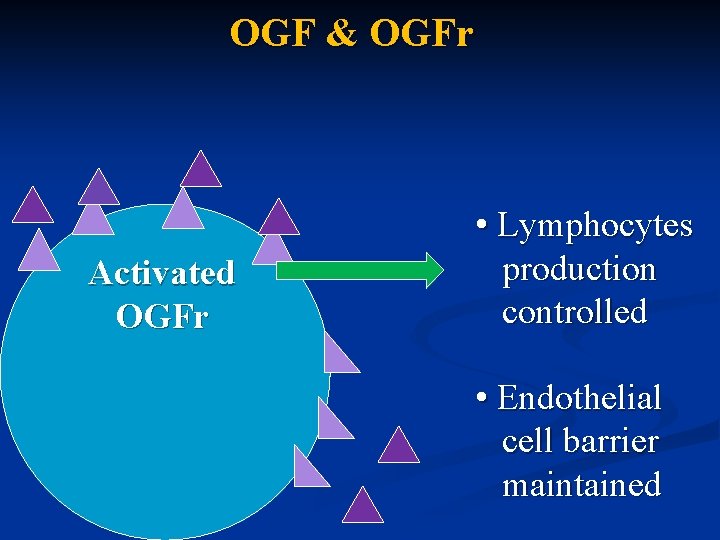
OGF & OGFr Activated OGFr • Lymphocytes production controlled • Endothelial cell barrier maintained

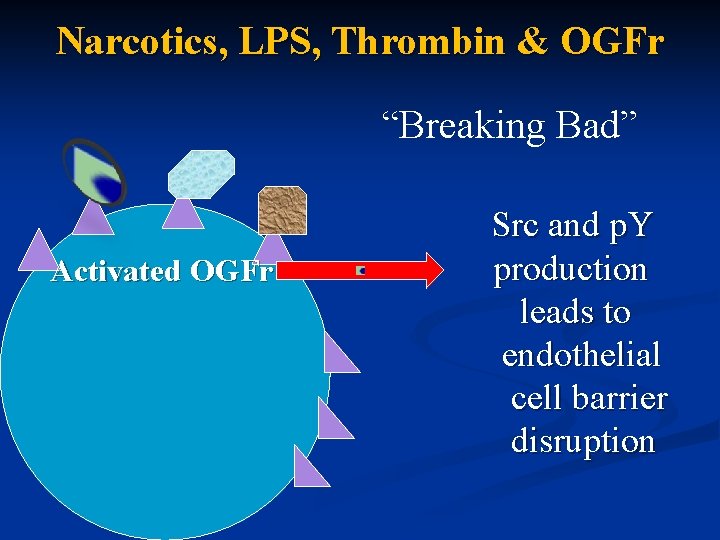
Narcotics, LPS, Thrombin & OGFr “Breaking Bad” Src and p. Y Activated OGFr production leads to endothelial cell barrier disruption

LDN MOA n Naltrexone displaces endorphins from OGFr n Cells become deficient in OGFr production increased n OGFr sensitivity increased

LDN & OGFr Decreased OGFr Activity Short-term Cells perceive OGFr reduction

LDN & OGFr Activated OGFr More OGF and OGFr lead to decreased T- and B-cell activity and less permeability

LDN MOA n LDN blocks the OGF receptors only for a few hours – leads to a rebound effect; in which both the production and utilization of OGF is greatly increased. n Endorphins now interact with the more-sensitive and more-plentiful receptors and assist in regulating cell

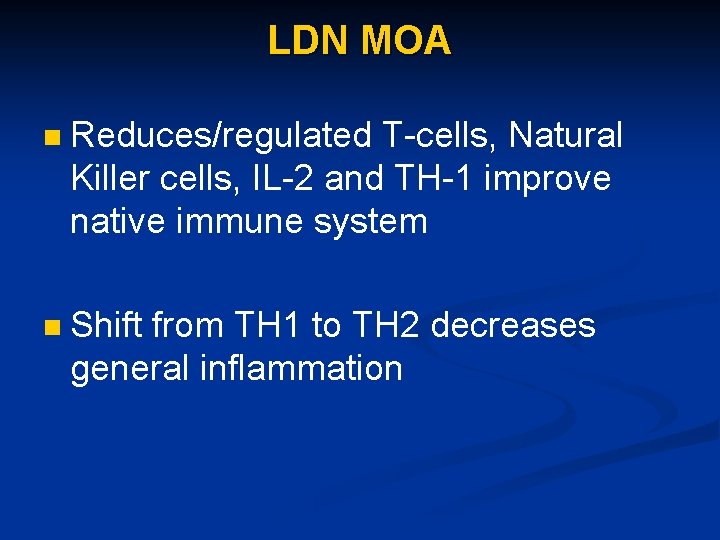
LDN MOA n Reduces/regulated T-cells, Natural Killer cells, IL-2 and TH-1 improve native immune system n Shift from TH 1 to TH 2 decreases general inflammation

Additional MOA – Neuroreceptors n Two opioid receptors systems with opposite effects: low and high affinity receptors Activation of each affected differently by different concentrations of opioids n Bimodal excitatory and inhibitory opioid receptor function (only excitatory system is blocked by LDN) n n Various disease states (e. g. , IBS) may have altered pathway predominance and altered endogenous opioid production Kariv. Dig Dis Sci 2006; 51: 2128 -33.

LDN & excitatory nerve function LDN activates excitatory receptors of nerve Endorphins act on inhibitory receptors of sensory nerves

Additional MOA – Toll receptors n Endothelial receptors – possible MOA for IBD GI receptor allows for increase in bacterial translocation – exacerbated by exogenous opioids n LDN may stabilize receptor and decrease bacterial translocation n n Glial receptor Activated microglia cause neuroexitability and enhanced pain via toll-like receptor 4 pathway n LDN antagonizes pathway Li. Med Hypotheses 2012; 79: 754 -6. n Hutchinson et al. Brain Behav Immun 2010; 24: 83 -95.

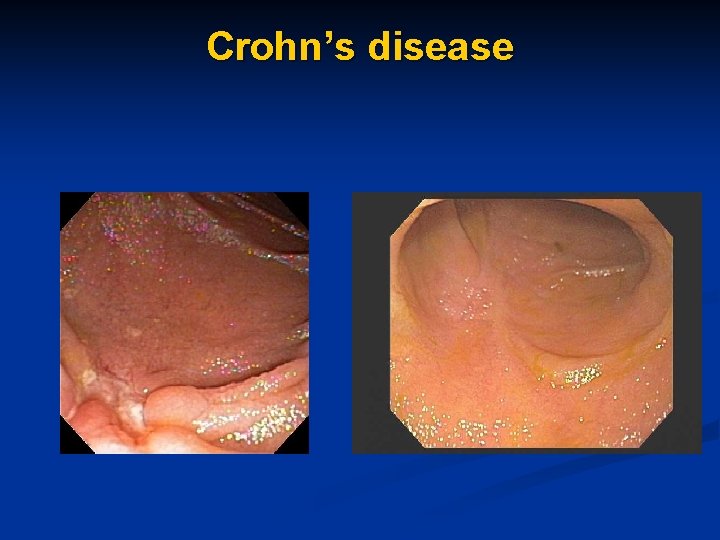
Crohn’s disease

Crohn’s disease – open label studies • Smith. LDN therapy improves active Crohn's disease. Am J Gastroenterol 2007; 102: 820 -828. • Shannon. LDN for treatment of duodenal Crohn’s disease in a pediatric patient. Inflamm Bowel Dis 2010; 16: 1457.

Crohn’s disease • Open label study: 4. 5 mg LDN in moderate to severe CD (N=33 adults) • Failing 5 -ASA followed by 6 -MP and/or IFX • LDN Rx: 40 ± 43 wks (max 200 wks) • 5 withdrew - AE (mild-moderate) • Positive clinical response in 15/33 pts • 11 of 15 responders: C-scope before and after Rx: 8 complete healing, 1 partial healing and 2 unchanged Weinstock. J Clin Gastro 2014

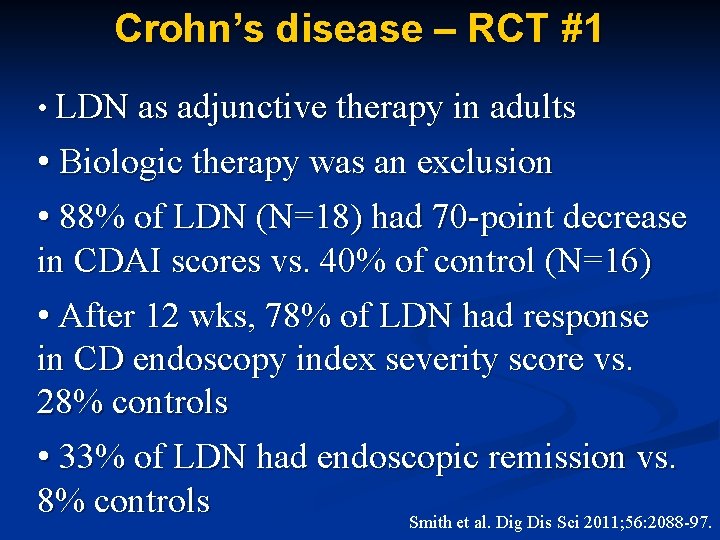
Crohn’s disease – RCT #1 • LDN as adjunctive therapy in adults • Biologic therapy was an exclusion • 88% of LDN (N=18) had 70 -point decrease in CDAI scores vs. 40% of control (N=16) • After 12 wks, 78% of LDN had response in CD endoscopy index severity score vs. 28% controls • 33% of LDN had endoscopic remission vs. 8% controls Smith et al. Dig Dis Sci 2011; 56: 2088 -97.

Crohn’s disease – RCT #2 • LDN as sole therapy in 14 children • LDN (0. 1 mg/kg) vs. placebo for 8 wks • CDAI: 34± 3 decreased to 22± 4 CDAI: (P=0. 005) and 25% went into remission • No serious adverse events Jill Smith et al. J Clin Gastroenterol 2013; 47: 339 -345.

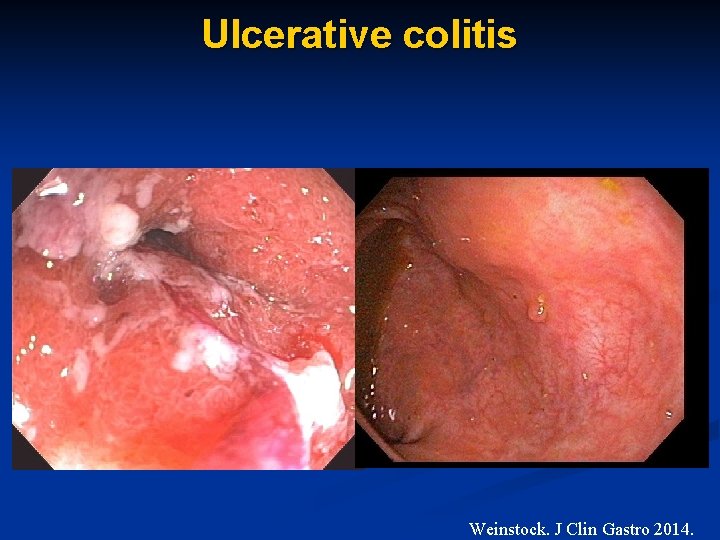
Ulcerative colitis Weinstock. J Clin Gastro 2014.

Ulcerative colitis – St. Louis • Open label study: 4. 5 mg LDN in moderate to severe UC (N=12) • Failing 5 -ASA followed by 6 -MP and/or IFX • LDN Rx: 46 ± 75 wks (max 270 wks) • 1 withdrew d/t insomnia • Positive clinical response in 6/12 pts • 2 of 6 responders: C-scope before and after Rx • 2 complete healing Weinstock 2013.

Irritable bowel syndrome n N=42 IBS n Open-label LDN 0. 5 mg/day/4 wks n Evaluation every wk n Pain-free days and global scores n Global improvement in 76% n Weekly # pain-free days increased from 0. 5/-1 to 1. 25+/2. 14 (P=0. 011) Kariv. Dig Dis Sci 2006; 51: 2128 -33.

Idiopathic IBS – St. Louis preliminary experience n N=13 IBS; Open-label Rx 2. 5 mg/day n IBS-d 3; IBS-a 5; IBS-c n 2 markedly improved n 5 moderately improved n 2 unchanged n 3 markedly worse Ploesser J, Weinstock LB, Thomas E. Internat J Pharm Compound 2010: 171 -173.

IBS-SIBO – St. Louis preliminary report n N=85 IBS; Open-label Rx 58 w 2. 5 mg/day and 27 w 2. 5 mg BID after antibiotic therapy n 18% markedly improved n 38% moderately improved n 11% mildly improve n 27% unchanged Ploesser J, Weinstock LB, Thomas E. Internat J Pharm Compound 2010: 171 -173. n Rest worse

Chronic constipation – St. Louis preliminary report n N=12; Open-label Rx 2. 5 mg twice a day n 58% markedly improved n 1% moderately improved n 25% mildly improve n 1% unchanged Ploesser J, Weinstock LB, Thomas E. Internat J Pharm Compound 2010: 171 -173.

Multiple Sclerosis: 1 st study • 6 mo trial in 40 pts • 1 o end points: safety and tolerability • 2 o outcomes: efficacy on spasticity, pain, fatigue, depression and QOL • 5 dropouts and 2 major AEs • Significant reduction of spasticity resulted • Disability progressed in 1 • Beta-endorphins increased during trial Gironi et al. Mult Scler 2008; 14: 1076 -83.

Multiple Sclerosis: RTC - Mental QOL • DB, PC, double-masked, X-over study: 8 wks 4. 5 mg/q. HS LDN on QOL • N=80 • 20 drop outs - multiple reasons (not AE) Cree et al. Ann Neuro 2010; 68: 145 -50.

MS Mental QOL study (cont. ) • Mental component general health survey: 3. 3 -pt improvement (p = 0. 04) • Mental health inventory: 6 -pt improvement (p < 0. 01) • Pain effects scale: 1. 6 -pt improvement (p =. 04) • Perceived deficits questionnaire: 2. 4 -pt improvement (p = 0. 05) • LDN improved MS QOL. Subject dropout . reduced statistical power

Multiple Sclerosis QOL: RTC #2 • • 17 -week R, DB, PC parallel-group, crossover : 96 pts with relapsing-remitting or secondary progressive disease • Primary outcome: scores of physical & mental health obtained in the middle and end of study Sharafaddinzadeh et al. Mult Scler. 2010; 16: 964 -9

LDN and MS QOL: study #2 (cont. ) • No diff in pain, energy, emotional wellbeing, social, cognitive, sexual functions, role limitation due to physical and emotional problems, health distress • LDN is a relatively safe therapeutic option but efficacy is under question and probably a long duration trial is needed in the future Sharafaddinzadeh et al. Mult Scler. 2010; 16: 964 -9

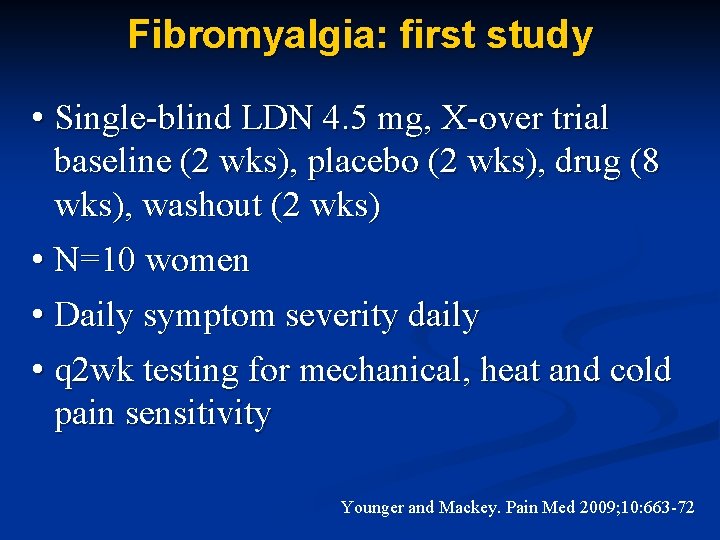
Fibromyalgia: first study • Single-blind LDN 4. 5 mg, X-over trial baseline (2 wks), placebo (2 wks), drug (8 wks), washout (2 wks) • N=10 women • Daily symptom severity daily • q 2 wk testing for mechanical, heat and cold pain sensitivity Younger and Mackey. Pain Med 2009; 10: 663 -72

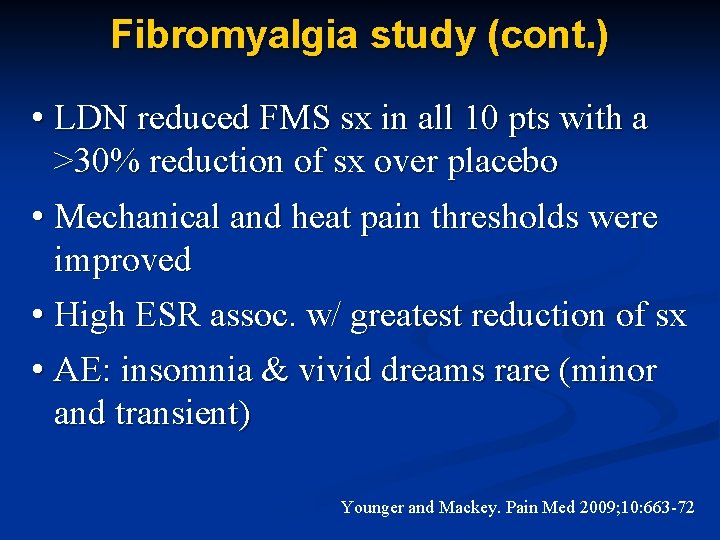
Fibromyalgia study (cont. ) • LDN reduced FMS sx in all 10 pts with a >30% reduction of sx over placebo • Mechanical and heat pain thresholds were improved • High ESR assoc. w/ greatest reduction of sx • AE: insomnia & vivid dreams rare (minor and transient) Younger and Mackey. Pain Med 2009; 10: 663 -72

Fibromyalgia: RTC study • LDN 4. 5 mg/day vs. placebo • N=31 women • Randomized, double-blind, placebocontrolled, counterbalanced, x-over study. • Questionnaires to measure daily levels of pain. Secondary outcomes included general satisfaction with life, positive mood, sleep quality and fatigue Younger et al. Arthritis Rheum 2013 Feb; 65: 52938.

Fibromyalgia: RTC study (cont. ) • 28. 8% pain reduction with LDN vs. 18. 0% reduction with placebo (P = 0. 016) • LDN improved general satisfaction with life (P = 0. 045) and improved mood (P = 0. 039) • 32% had a significant reduction in pain plus a significant reduction in either fatigue or sleep problems vs. 11% response rate with placebo (P = 0. 05) • LDN equally tolerable as placebo. No serious side effects were reported Younger et al. Arthritis Rheum 2013 Feb; 65: 529 -

Complex Regional Pain Syndrome • Reflex Sympathetic Dystrophy or Neurovascular Dystrophy • Severe pain, swelling & changes in extremities • Spreads throughout the body in 92% • Neurogenic inflammation, pain sensitization vasomotor dysfx & aberrant response to tissue injury

Complex Regional Pain Syndrome Report: 2 cases with improvement with LDN Chopra. Neuroimmune Pharmacol 2013; 8: 470 -6.

Complex Regional Pain Syndrome 42 y. o. WF 12 yr Hx of CRPS pain, flushing, shiny painful skin Failed gabatenam and sympathectomy Narcotics made pain worse (8 Viocodin/d)

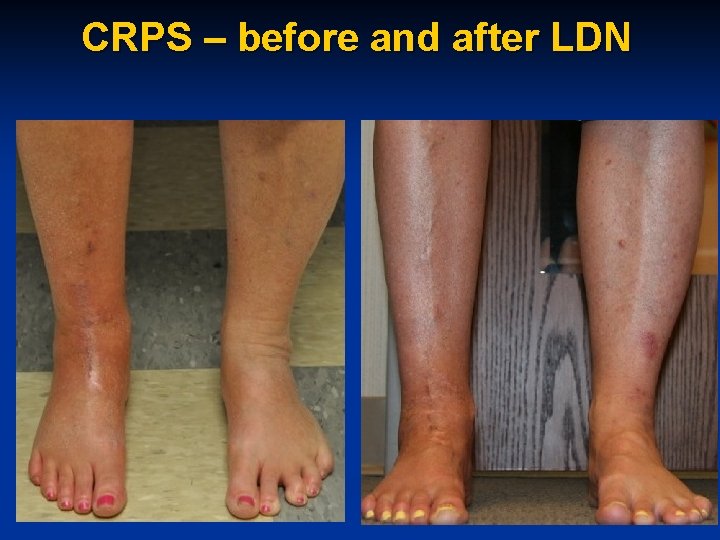
CRPS – before and after LDN

40 y. o. WF w CRPS n Currently on alprazolam, Wellbutrin 450 mg and occ Tramadol n 4. 5 mg LDN prescribed n “I am thrilled as I usually have more CRPS pain with weather changes. It snowed here in Indiana and I have minimal pain which is strangely wonderful “

Cancer and LDN Publications by Zagon et al. General changes in cancer cells • Ovarian cancer • Breast cancer • Head and neck cancer • Prostate cancer

Basic science studies of cancer • Donahue RN. The opioid growth factor (OGF) and low dose naltrexone (LDN) suppress human ovarian cancer progression in mice. Gynecol Oncol 2011; 122: 382 -8. • Donahue RN. Cell proliferation of human ovarian cancer is regulated by the opioid growth factor-opioid growth factor receptor axis. J Physiol Regul Integr Comp Physiol. 2009; 296: R 1716 -25. • Avella DM. The opioid growth factor-opioid growth factor receptor axis regulates cell proliferation of human hepatocellular cancer. Am J Physiol Regul Integr Comp Physiol. 2010; 298: R 459 -66. • Mc. Laughlin PJ. Growth inhibition of thyroid follicular cell-derived cancers by the opioid growth factor (OGF) - opioid growth factor receptor (OGFr) axis. BMC Cancer. 2009; 9: 369 • Mc. Laughlin PJ. Modulation of the opioid growth factor ([Met(5)]enkephalin)-opioid growth factor receptor axis: novel therapies for squamous cell carcinoma of the head and neck. Head Neck 2012; 34: 5139. • Zagon IS. Opioid growth factor - opioid growth factor receptor axis inhibits proliferation of triple negative breast cancer. Exp Biol Med

Cancer tissue model study • Opioid receptor blockade in ovarian CA cells • Short-term Met(5)]-enkephalin and receptor responsible for MAO of NTX on cell proliferation • NTX up-regulated OGF and OGFr at translational level • Required p 16 and/or p 21 cyclin-dependent inhibitory kinases • Not dependent on cell survival (necrosis, apoptosis) Donahue RN, Mc. Laughlin PJ, Zagon IS. Exp Biol Med 2011; 236: 1036 -50.

Pancreatic Cancer and Lymphoma n Pancreatic cancer – combined in 3 pts with I. V. alpha-lipoic acid - stabilization and/or regression of metastatic disease (4, 5 and 39 months) n One of the 3 patients also had reversal of signs and symptoms concomittant B-cell lymphoma Berkson et al. Integr Cancer Ther. 2007; 6: 293 -6.

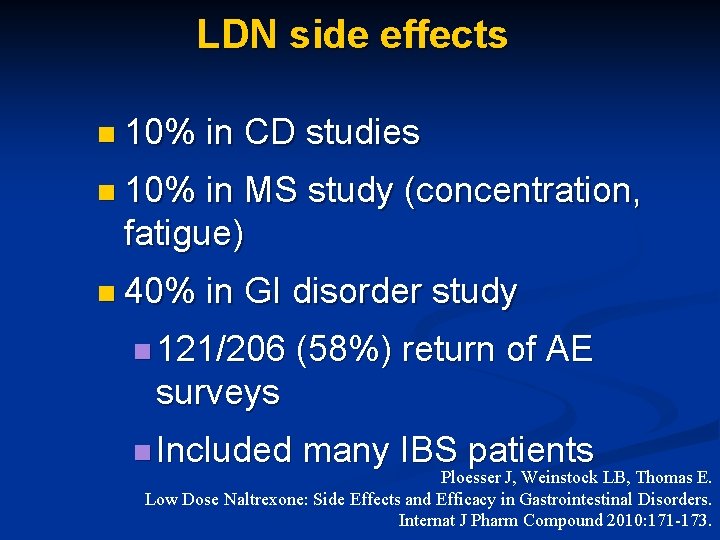
LDN side effects n 10% in CD studies n 10% in MS study (concentration, fatigue) n 40% in GI disorder study n 121/206 (58%) return of AE surveys n Included many IBS patients Ploesser J, Weinstock LB, Thomas E. Low Dose Naltrexone: Side Effects and Efficacy in Gastrointestinal Disorders. Internat J Pharm Compound 2010: 171 -173.

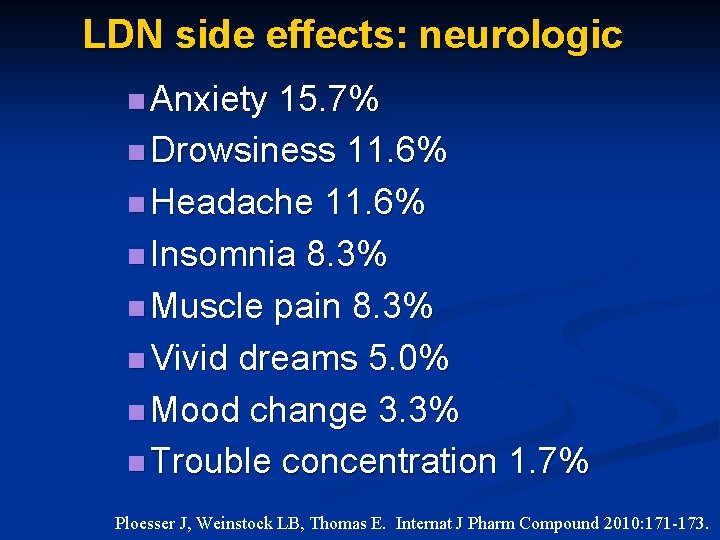
LDN side effects: neurologic n Anxiety 15. 7% n Drowsiness 11. 6% n Headache 11. 6% n Insomnia 8. 3% n Muscle pain 8. 3% n Vivid dreams 5. 0% n Mood change 3. 3% n Trouble concentration 1. 7% Ploesser J, Weinstock LB, Thomas E. Internat J Pharm Compound 2010: 171 -173.

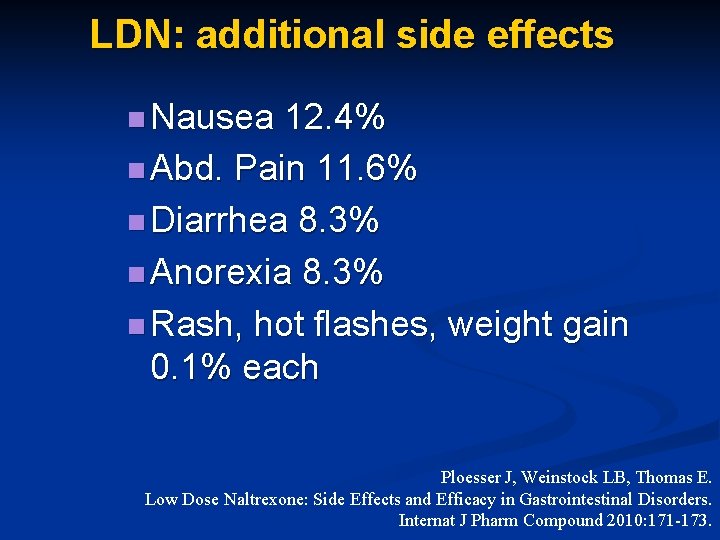
LDN: additional side effects n Nausea 12. 4% n Abd. Pain 11. 6% n Diarrhea 8. 3% n Anorexia 8. 3% n Rash, hot flashes, weight gain 0. 1% each Ploesser J, Weinstock LB, Thomas E. Low Dose Naltrexone: Side Effects and Efficacy in Gastrointestinal Disorders. Internat J Pharm Compound 2010: 171 -173.

Conclusions n “Breaking Bad”: Endorphins good, Narcotics bad n High quality studies are needed for LDN n Methyl-naltrexone is a promising treatment for several GI and SIBO related conditions n Methyl-naltrexone should have lower AE profile but does not cross BBB so it may not be effective for all MORA-
 Macrobid cost walgreens
Macrobid cost walgreens Naltrexone and autism
Naltrexone and autism Breaking bad
Breaking bad Naltrexone black box warning
Naltrexone black box warning Increase bp
Increase bp Standard screening tomohd
Standard screening tomohd Reflective style of communication
Reflective style of communication Aspirin action
Aspirin action Mechanism of action of asprin
Mechanism of action of asprin Mid = low + (high - low) / 2
Mid = low + (high - low) / 2 Low accuracy low precision
Low accuracy low precision Low voltage = low hazard
Low voltage = low hazard Tools and mechanisms of virtualization
Tools and mechanisms of virtualization Circular motion examples
Circular motion examples Magnetic read and write mechanisms
Magnetic read and write mechanisms Basic mechanisms underlying seizures and epilepsy
Basic mechanisms underlying seizures and epilepsy Sensory vs motor
Sensory vs motor Security services and mechanisms in cryptography
Security services and mechanisms in cryptography Internal and external service delivery mechanisms
Internal and external service delivery mechanisms Security attacks services and mechanisms
Security attacks services and mechanisms Chapter 11 health vocabulary
Chapter 11 health vocabulary National mechanisms for reporting and follow-up
National mechanisms for reporting and follow-up It is the organized sequence
It is the organized sequence What is exposition in a story
What is exposition in a story Stages of the plot
Stages of the plot Exposition rising action climax falling action resolution
Exposition rising action climax falling action resolution Suit the action to the word the word to the action meaning
Suit the action to the word the word to the action meaning Medium dose ics and laba
Medium dose ics and laba Ionizing radiation examples
Ionizing radiation examples Benzodiazepine dose and route
Benzodiazepine dose and route Benzodiazépines
Benzodiazépines Loading dose formula in pharmacology
Loading dose formula in pharmacology Mechanisms of movement lab report
Mechanisms of movement lab report What are freud's defense mechanisms
What are freud's defense mechanisms Countercurrent exchange thermoregulation
Countercurrent exchange thermoregulation Neutral thermal environment chart
Neutral thermal environment chart Freud defense mechanism examples
Freud defense mechanism examples What are the first line of defense
What are the first line of defense Level of consciousness freud
Level of consciousness freud How does a sponge defend itself
How does a sponge defend itself Pay-per-use monitor
Pay-per-use monitor Secure attachment style
Secure attachment style Types of pop up card mechanisms
Types of pop up card mechanisms Reaction formation
Reaction formation Main personality traits
Main personality traits Ego defense mechanisms
Ego defense mechanisms Free association psychology
Free association psychology Prolapsed umbilical cord
Prolapsed umbilical cord Ap psychology defense mechanisms worksheet answers
Ap psychology defense mechanisms worksheet answers 3 mechanisms of microevolution
3 mechanisms of microevolution Portal circulation
Portal circulation Mechanisms of evolution
Mechanisms of evolution Natural selection vs evolution
Natural selection vs evolution Lever linkage mechanisms
Lever linkage mechanisms Vex lifting mechanisms
Vex lifting mechanisms What are defense mechanisms according to freud?
What are defense mechanisms according to freud? Synectic triggers examples
Synectic triggers examples The real reason dinosaurs became extinct
The real reason dinosaurs became extinct Conduction heat loss newborn
Conduction heat loss newborn