Aim What factors affect rate of reactions DO








- Slides: 14

Aim: What factors affect rate of reactions? DO NOW: 1. What does the word “rate” mean? 2. What would affect the rate of reaction? What would speed up a reaction?

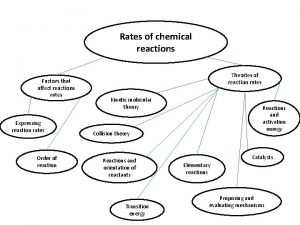
Terminology • Rate of reaction- how fast, how many moles are produced or consumed in a unit of time • Collision Theory- rate of reaction depends on how often collisions occur and how many of them are effective • Activation Energy- smallest amount of energy needed to start a reaction

Collision Theory For a reaction to happen, the particles must have effective collision; particles must have enough energy and collide at the correct angles. The collision theory explains the factors that affect the rate of reactions.



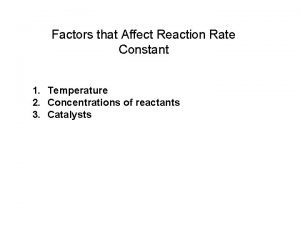
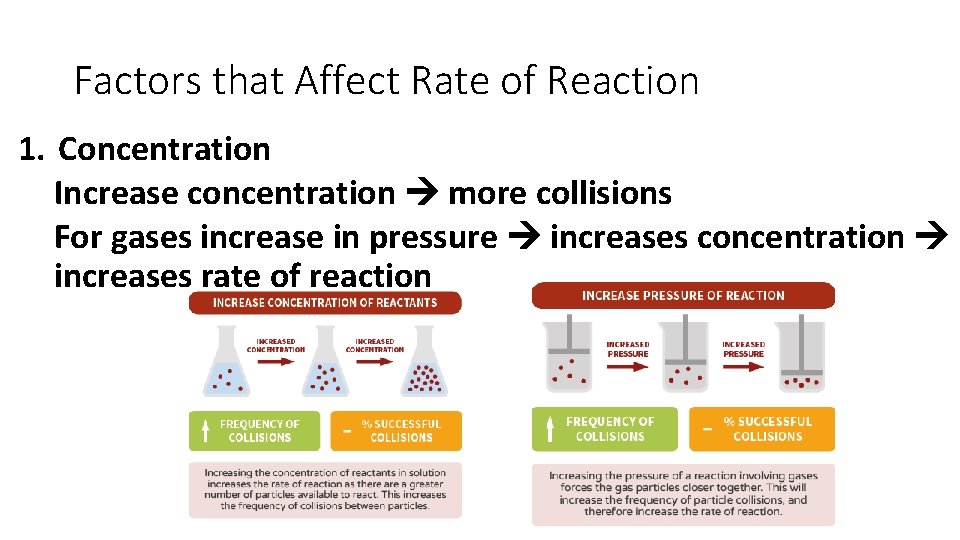
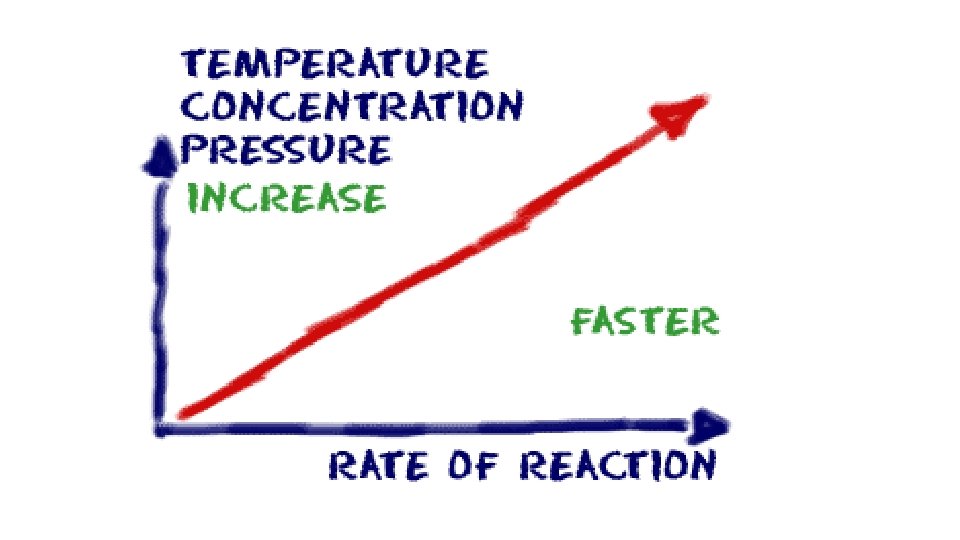
Factors that Affect Rate of Reaction 1. Concentration Increase concentration more collisions For gases increase in pressure increases concentration increases rate of reaction

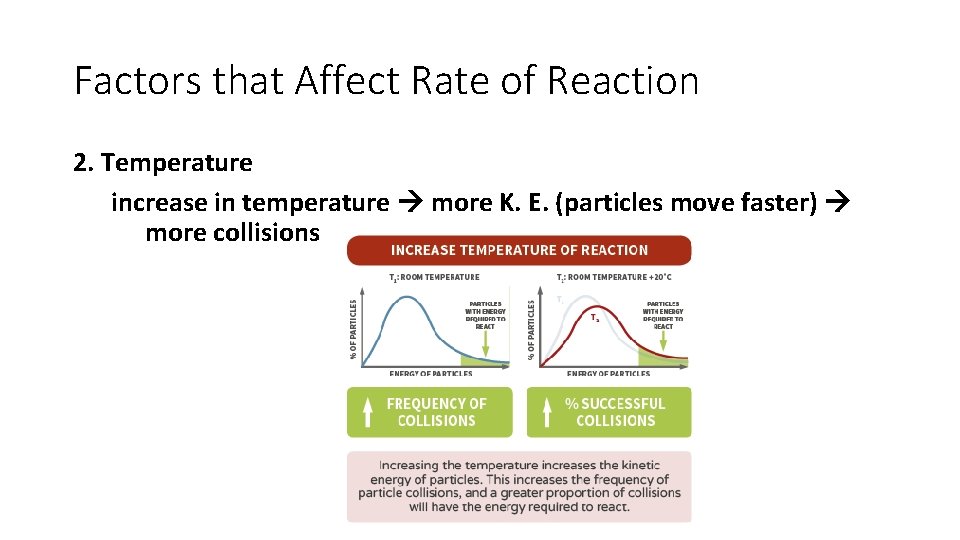
Factors that Affect Rate of Reaction 2. Temperature increase in temperature more K. E. (particles move faster) more collisions

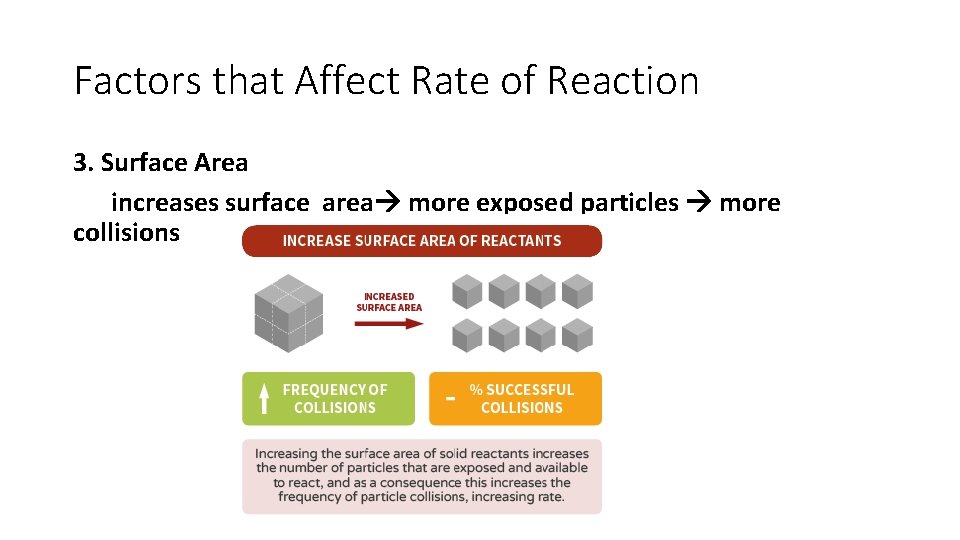
Factors that Affect Rate of Reaction 3. Surface Area increases surface area more exposed particles more collisions

Factors that Affect Rate of Reaction 4. Nature of Reactants Ionic substances react faster than molecules; involve less rearrangement during a reaction.

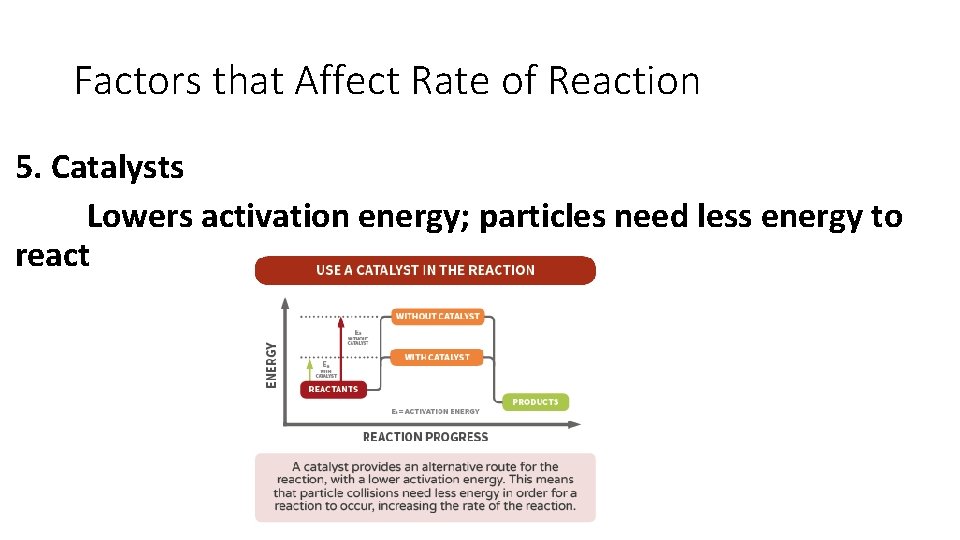
Factors that Affect Rate of Reaction 5. Catalysts Lowers activation energy; particles need less energy to react


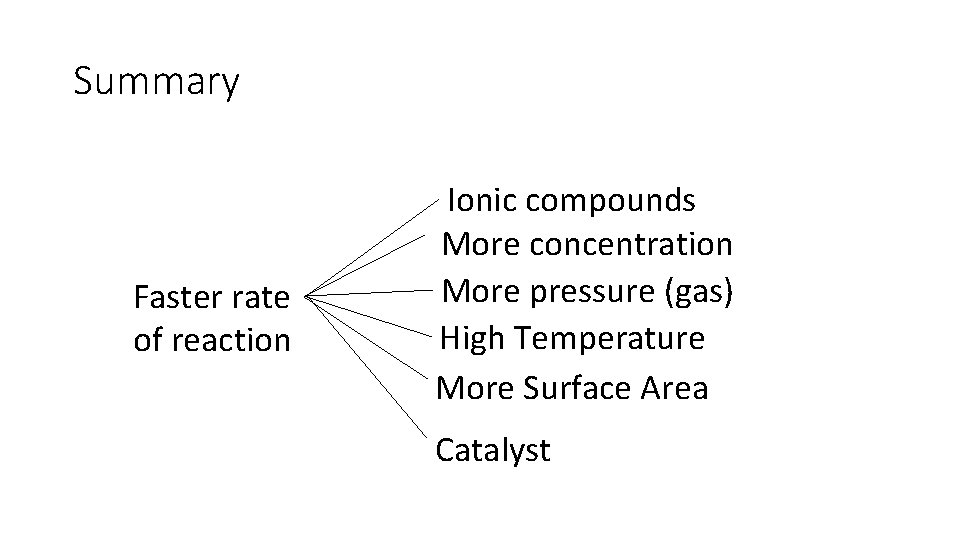
Summary Faster rate of reaction Ionic compounds More concentration More pressure (gas) High Temperature More Surface Area Catalyst

1. Consider the following reaction. Mg(s) + 2 H 2 O (l) --> Mg(OH)2 + H 2(g) For the reaction to occur at the fastest rate, 1 g of Mg (s) should be added in the form of 1) large chunks 3) a ribbon 2) small chunks 4) a powder 2. Raising the temperature speeds up the rate of chemical reaction by increasing 1) the effectiveness of the collisions only 2) the frequency of the collisions only 3) both the effectiveness and frequency of the collisions 4) neither the effectiveness nor frequency of the collisions

3. Consider the following equation. Fe(s) + Cu. SO 4(aq) --> Cu(s) + Fe. SO 4(aq) The Fe reacts more rapidly when it is powdered because the increased surface due to powdering permits 1) increased reactant contact 2) decreased reactant contact 3) pressure to affect reaction rate 4) warmer solution to be used 4. If the pressure on gaseous reactants is increased the rate of the reaction is increased because there is an increase in the 1) temperature 2) volume 3) concentration 4) heat of reaction
 Factors that affect the rate of reactions
Factors that affect the rate of reactions Maylon hsu md
Maylon hsu md Diffusion in gelatin
Diffusion in gelatin What factors affect the rate of corrosion
What factors affect the rate of corrosion Factors affecting rate constant
Factors affecting rate constant Difference between enzyme and protein
Difference between enzyme and protein The enzyme would most likely affect reactions involving
The enzyme would most likely affect reactions involving Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Redox reactions half reactions
Redox reactions half reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Grace brancale
Grace brancale How did the minie-ball affect the casualty rate of the war?
How did the minie-ball affect the casualty rate of the war? Maxwell boltzmann distribution catalyst
Maxwell boltzmann distribution catalyst