Aim What factors can affect the rate of




- Slides: 11

Aim: What factors can affect the rate of a chemical reaction? Do Now: 1. Take out a calculator and reference tables. 2. What does the word kinetic mean?

Collision Theory • For a reaction to occur, particles must collide in a specific manner. • Factors affecting rates of collision include: 1. Nature of the reactants 2. Concentration 3. Surface Area 4. Pressure 5. The presence of a catalyst 6. Temperature

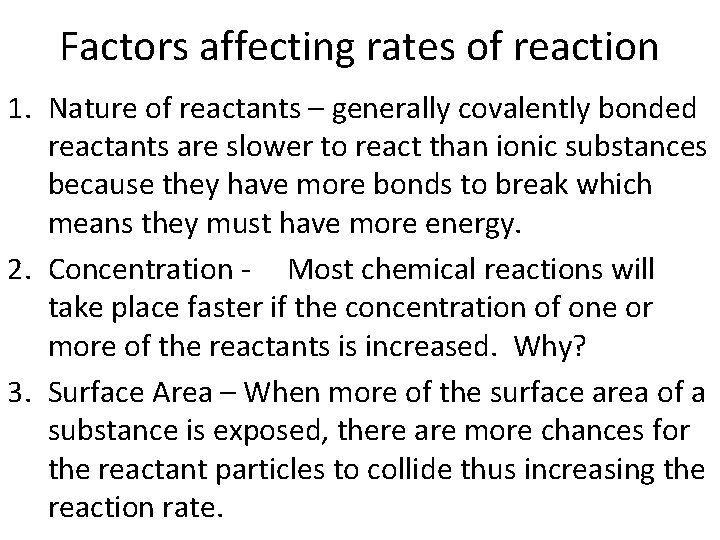
Factors affecting rates of reaction 1. Nature of reactants – generally covalently bonded reactants are slower to react than ionic substances because they have more bonds to break which means they must have more energy. 2. Concentration - Most chemical reactions will take place faster if the concentration of one or more of the reactants is increased. Why? 3. Surface Area – When more of the surface area of a substance is exposed, there are more chances for the reactant particles to collide thus increasing the reaction rate.

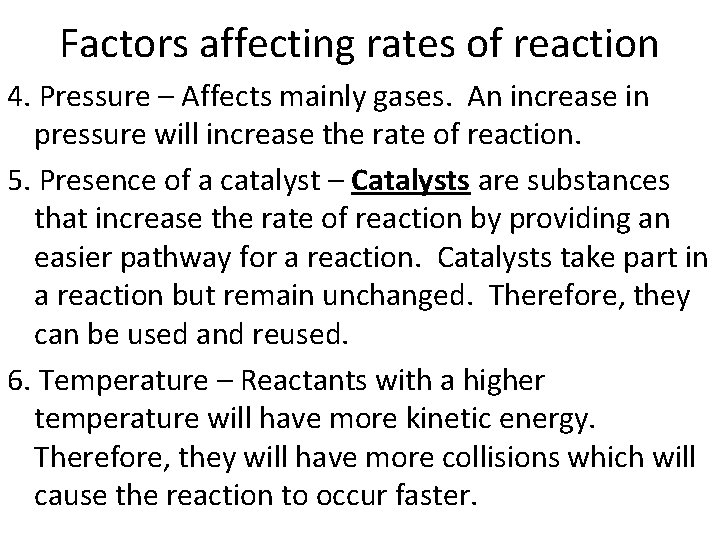
Factors affecting rates of reaction 4. Pressure – Affects mainly gases. An increase in pressure will increase the rate of reaction. 5. Presence of a catalyst – Catalysts are substances that increase the rate of reaction by providing an easier pathway for a reaction. Catalysts take part in a reaction but remain unchanged. Therefore, they can be used and reused. 6. Temperature – Reactants with a higher temperature will have more kinetic energy. Therefore, they will have more collisions which will cause the reaction to occur faster.

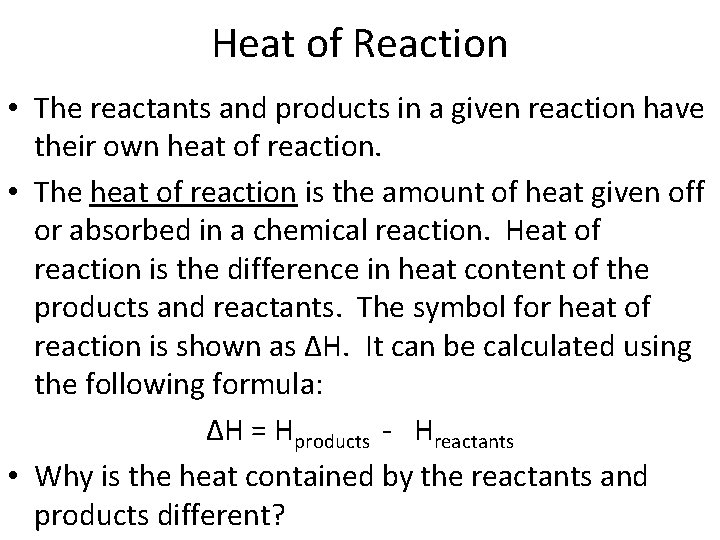
Heat of Reaction • The reactants and products in a given reaction have their own heat of reaction. • The heat of reaction is the amount of heat given off or absorbed in a chemical reaction. Heat of reaction is the difference in heat content of the products and reactants. The symbol for heat of reaction is shown as ∆H. It can be calculated using the following formula: ∆H = Hproducts - Hreactants • Why is the heat contained by the reactants and products different?

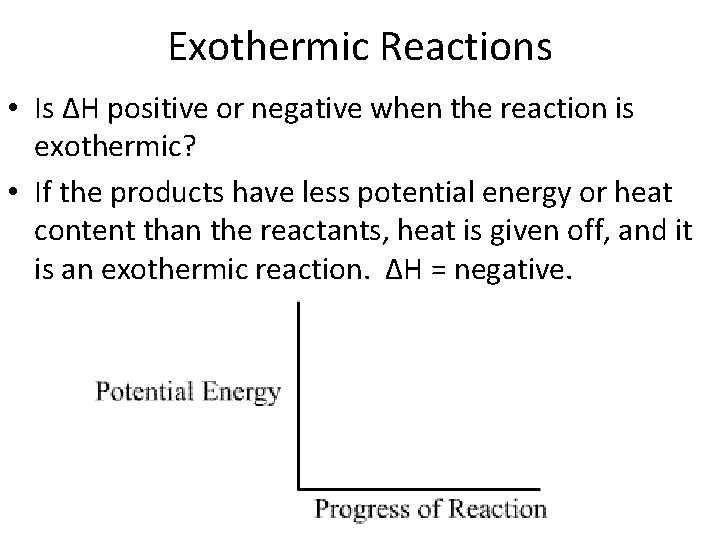
Exothermic Reactions • Is ∆H positive or negative when the reaction is exothermic? • If the products have less potential energy or heat content than the reactants, heat is given off, and it is an exothermic reaction. ∆H = negative.

Endothermic Reactions • Is ∆H positive or negative when the reaction is endothermic? • If the products have more potential energy or heat content than the reactants, heat is absorbed, and it is an endothermic reaction. ∆H = positive.

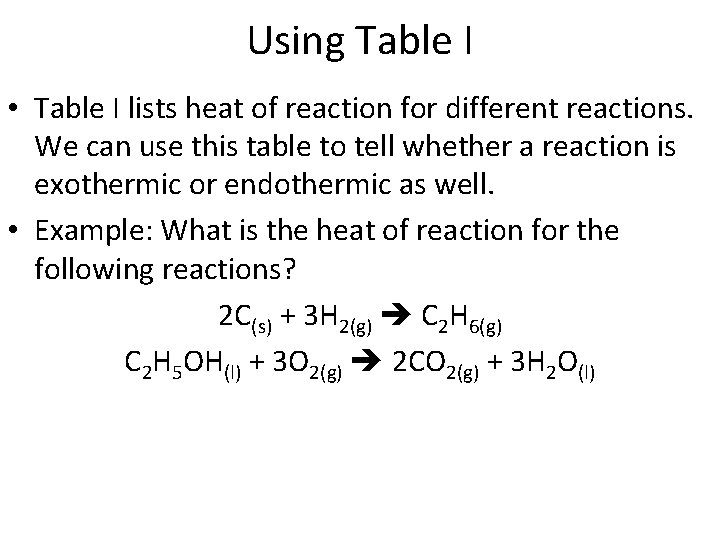
Using Table I • Table I lists heat of reaction for different reactions. We can use this table to tell whether a reaction is exothermic or endothermic as well. • Example: What is the heat of reaction for the following reactions? 2 C(s) + 3 H 2(g) C 2 H 6(g) C 2 H 5 OH(l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(l)

Activation energy • Activation energy is the smallest amount of energy needed to form an activated complex. • What’s an activated complex? • An activated complex is the result of a collision between the reactants in a chemical reaction. It is temporary and will either break apart and reform the reactants or rearrange and form new products.

Parts of a Potential Energy Diagram

Activity/Homework • Review Book pages 137 -141. Copy and answer question 1 -17.