Aim 46 How does a solute affect the



- Slides: 12

Aim # 46: How does a solute affect the vapor pressure of a solvent? H. W. # 46 Study pp. 521 – 526(sec. 11. 4) Ans. ques. p. 521 # 51, 54, 59, 64

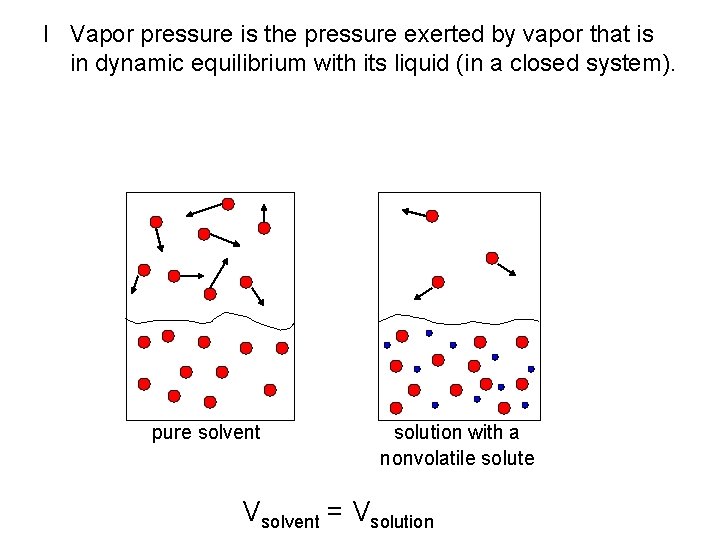
I Vapor pressure is the pressure exerted by vapor that is in dynamic equilibrium with its liquid (in a closed system). pure solvent solution with a nonvolatile solute Vsolvent = Vsolution

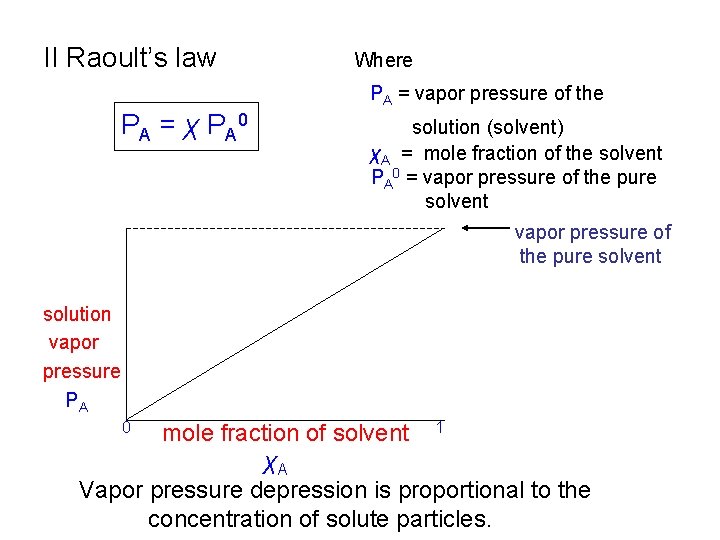
II Raoult’s law Where PA = vapor pressure of the P A = χ P A 0 solution (solvent) χA = mole fraction of the solvent PA 0 = vapor pressure of the pure solvent solution vapor pressure PA mole fraction of solvent 1 χA Vapor pressure depression is proportional to the concentration of solute particles. 0

Problem: Urea, (NH 3)2 CO, has practically no vapor pressure at ordinary temperatures. The vapor pressure of pure water at 30. 0 C is 31. 824 torr. A solution contains 50. 0 g urea and 1000. g H 2 O. Find the vapor pressure of this solution at 30. 0 C. Ans: nurea = 50. 0 g x 1 mol =. 833 mol 60. 0 g n. H 20 = 1000. 0 g x 1 mol = 55. 555 mol 18. 0 g PH 20 = χH 20 P 0 H 20 PH 20 = 55. 555. 833 + 55. 555 x 31. 824 torr = 31. 354 torr The vapor pressure is lower by 0. 470 torr.

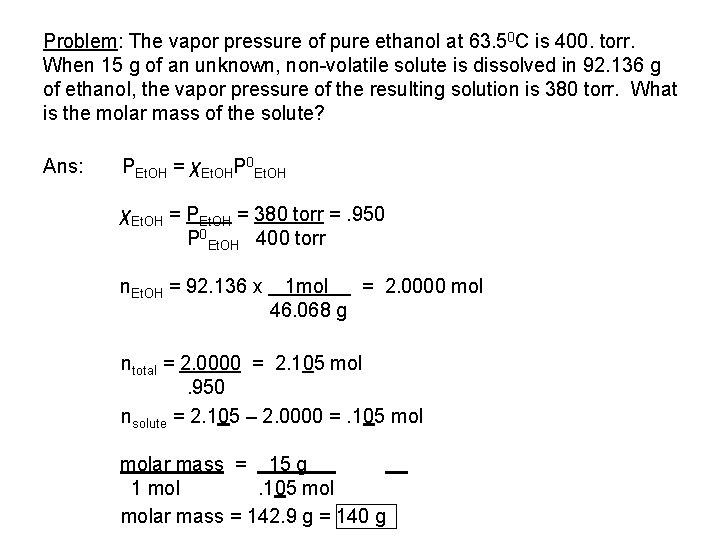
Problem: The vapor pressure of pure ethanol at 63. 50 C is 400. torr. When 15 g of an unknown, non-volatile solute is dissolved in 92. 136 g of ethanol, the vapor pressure of the resulting solution is 380 torr. What is the molar mass of the solute? Ans: PEt. OH = χEt. OHP 0 Et. OH χEt. OH = PEt. OH = 380 torr =. 950 P 0 Et. OH 400 torr n. Et. OH = 92. 136 x 1 mol = 2. 0000 mol 46. 068 g ntotal = 2. 0000 = 2. 105 mol. 950 nsolute = 2. 105 – 2. 0000 =. 105 molar mass = 15 g 1 mol. 105 molar mass = 142. 9 g = 140 g

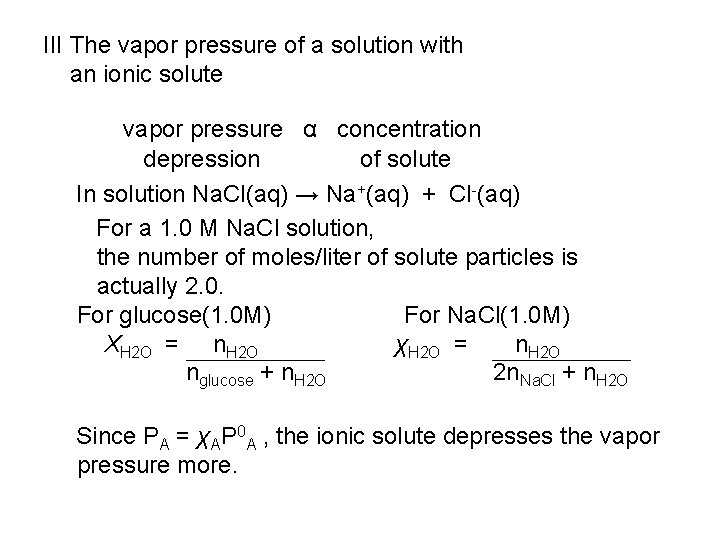
III The vapor pressure of a solution with an ionic solute vapor pressure α concentration depression of solute In solution Na. Cl(aq) → Na+(aq) + Cl-(aq) For a 1. 0 M Na. Cl solution, the number of moles/liter of solute particles is actually 2. 0. For glucose(1. 0 M) For Na. Cl(1. 0 M) XH 2 O = n. H 2 O χH 2 O = n. H 2 O nglucose + n. H 2 O 2 n. Na. Cl + n. H 2 O Since PA = χAP 0 A , the ionic solute depresses the vapor pressure more.

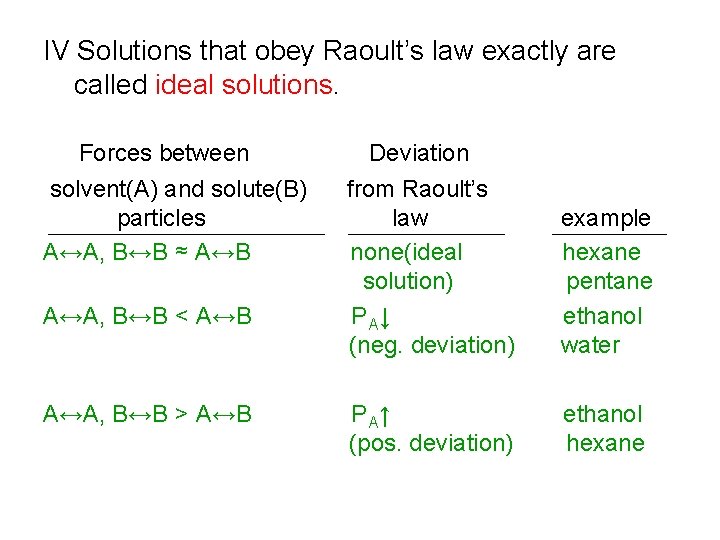
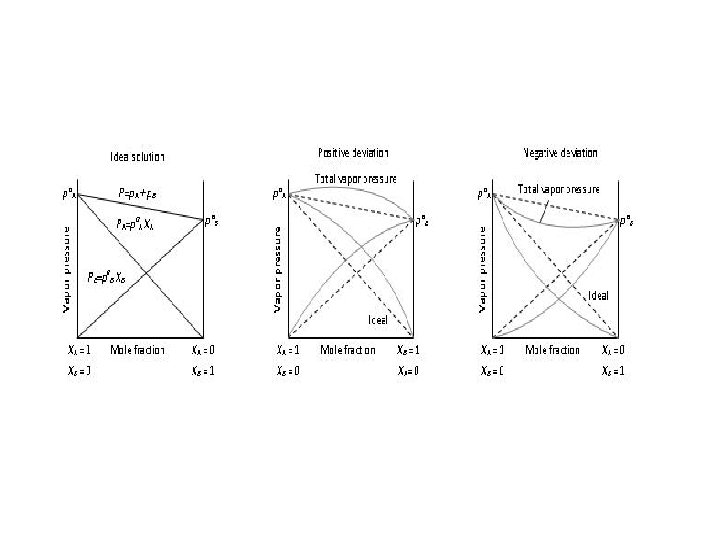
IV Solutions that obey Raoult’s law exactly are called ideal solutions. Forces between solvent(A) and solute(B) particles A↔A, B↔B ≈ A↔B A↔A, B↔B < A↔B A↔A, B↔B > A↔B Deviation from Raoult’s law none(ideal solution) P A↓ (neg. deviation) example hexane pentane ethanol water P A↑ (pos. deviation) ethanol hexane


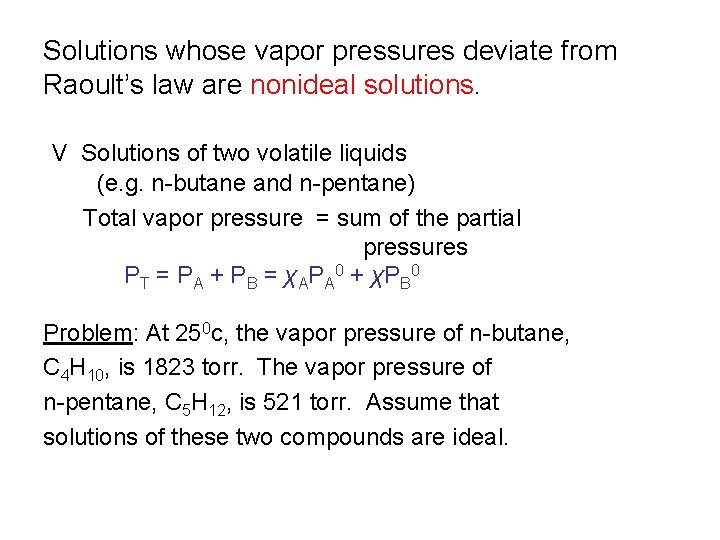
Solutions whose vapor pressures deviate from Raoult’s law are nonideal solutions. V Solutions of two volatile liquids (e. g. n-butane and n-pentane) Total vapor pressure = sum of the partial pressures PT = PA + PB = χAPA 0 + χPB 0 Problem: At 250 c, the vapor pressure of n-butane, C 4 H 10, is 1823 torr. The vapor pressure of n-pentane, C 5 H 12, is 521 torr. Assume that solutions of these two compounds are ideal.

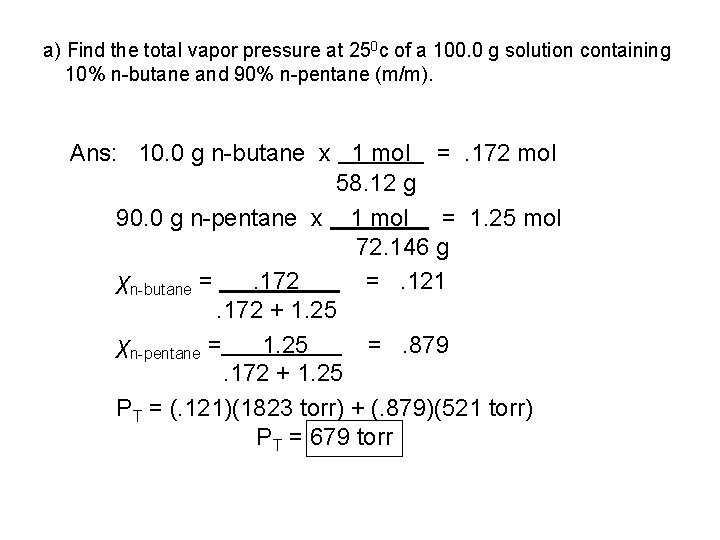
a) Find the total vapor pressure at 250 c of a 100. 0 g solution containing 10% n-butane and 90% n-pentane (m/m). Ans: 10. 0 g n-butane x 1 mol =. 172 mol 58. 12 g 90. 0 g n-pentane x 1 mol = 1. 25 mol 72. 146 g χn-butane =. 172 =. 121. 172 + 1. 25 χn-pentane = 1. 25 =. 879. 172 + 1. 25 PT = (. 121)(1823 torr) + (. 879)(521 torr) PT = 679 torr

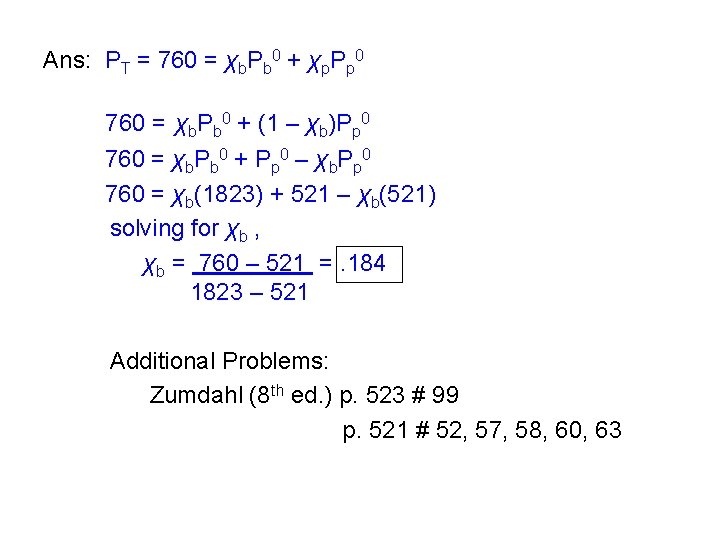
b) What is the mole fraction of the butane in the vapor? Ans: From Dalton’s law of partial pressures, Pn-butane = nn-butane = (. 121)(1823 torr) PT n. T 679 torr =. 325 c) Find the mole fraction of n-butane in a n-butane-n-pentane solution having a total vapor pressure of 760 torr at 250 C. Hint: χn-pentane = 1 – χn-butane

Ans: PT = 760 = χb. Pb 0 + χp. Pp 0 760 = χb. Pb 0 + (1 – χb)Pp 0 760 = χb. Pb 0 + Pp 0 – χb. Pp 0 760 = χb(1823) + 521 – χb(521) solving for χb , χb = 760 – 521 =. 184 1823 – 521 Additional Problems: Zumdahl (8 th ed. ) p. 523 # 99 p. 521 # 52, 57, 58, 60, 63