Access to essential medicines for NCDs WHO EMP







- Slides: 15

Access to essential medicines for NCDs WHO EMP and NVI Departments 1| Essential Medicines Technical Briefing Seminar

Access to NCD essential medicines on the global agenda l Access to chronic disease medicines is required for the fulfilment of MDG 8 – Governments, in collaboration with the private sector, should give greater priority to treating chronic diseases and improving the accessibility of medicines to treat them (MDG Report 2009) l Political declaration at the UN General Assembly, Sept 2011 (A/66. 1, 45 l) l WHA 2013: Endorsement of a Global Action Plan for the Prevention and Control of Non-communicable Diseases (NCDs) focusing on cardiovascular diseases, diabetes, CRDs and cancer including palliative care 2| Essential Medicines Technical Briefing Seminar

Global Action Plan and Monitoring Framework 3| Essential Medicines Technical Briefing Seminar

Target 9 l 80% availability of basic health technologies and essential medicines including generics required to treat major NCDs in both public and private facilities l Achieved in many LMICs for vaccines, TB and malaria medicines, ARVs l In 40 LMICs, availability of generic essential medicines in public and private sector (Cameron et al, 2011) – For treatment of acute communicable diseases: 53. 5% public, 66. 2% private – For chronic diseases: 36% public, 54. 7% private facilities l Costs of chronic medicine treatment can incur catastrophic health expenditure, pushing the family below the poverty line… however many of the commonly used medicines are out of patent and relatively cheap 4| Essential Medicines Technical Briefing Seminar

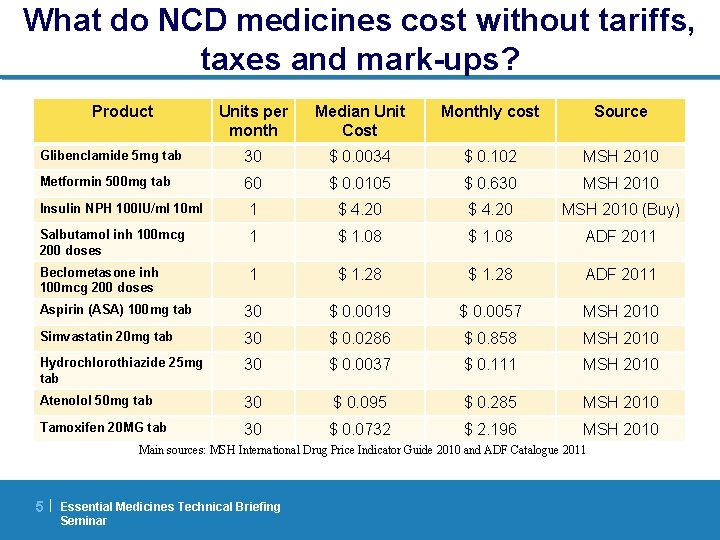
What do NCD medicines cost without tariffs, taxes and mark-ups? Product Units per month Median Unit Cost Monthly cost Source Glibenclamide 5 mg tab 30 $ 0. 0034 $ 0. 102 MSH 2010 Metformin 500 mg tab 60 $ 0. 0105 $ 0. 630 MSH 2010 Insulin NPH 100 IU/ml 10 ml 1 $ 4. 20 MSH 2010 (Buy) Salbutamol inh 100 mcg 200 doses 1 $ 1. 08 ADF 2011 Beclometasone inh 100 mcg 200 doses 1 $ 1. 28 ADF 2011 Aspirin (ASA) 100 mg tab 30 $ 0. 0019 $ 0. 0057 MSH 2010 Simvastatin 20 mg tab 30 $ 0. 0286 $ 0. 858 MSH 2010 Hydrochlorothiazide 25 mg tab 30 $ 0. 0037 $ 0. 111 MSH 2010 Atenolol 50 mg tab 30 $ 0. 095 $ 0. 285 MSH 2010 Tamoxifen 20 MG tab 30 $ 0. 0732 $ 2. 196 MSH 2010 Main sources: MSH International Drug Price Indicator Guide 2010 and ADF Catalogue 2011 5| Essential Medicines Technical Briefing Seminar

Challenges with NCD medicines l Oral medicines available as generic multisource products (Metformin, Aspirin, Hydrochlorothiazide, Tamoxifen) – cheap on the international market but not always available where patients need them, quality problems l Inhalers for asthma/COPD and insulin – available but more expensive and more sophisticated to produce and to use. For insulin, limited number of manufacturers, domination of the market by few pharmaceutical companies and specific conditions for distribution. l Some products still under patent and only accessible through large access programs from pharmaceutical companies, variable access for population. l Opioid analgesics: efficacious and at affordable costs, necessary for palliative care, not largely available due to legislative/regulatory barriers. 93, 8% of all licit morphine consumption by 21, 8% of the world population (INCB 2010, Data for 2009) 6| Essential Medicines Technical Briefing Seminar

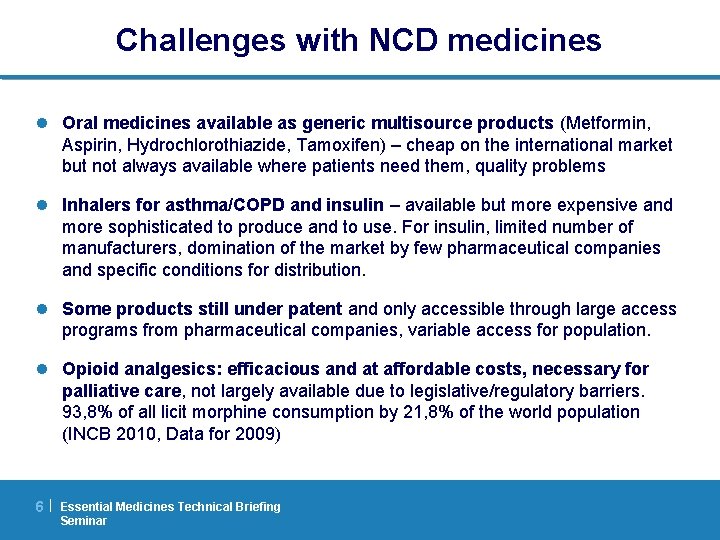
Availability of asthma inhalers Babar ZU, Lessing C, Mace C, Bissell K. The availability, pricing and affordability of three essential asthma medicines in 52 low- and middle income countries. Pharmacoeconomics. 2013; 31(11): 1063 -82. 7| Essential Medicines Technical Briefing Seminar

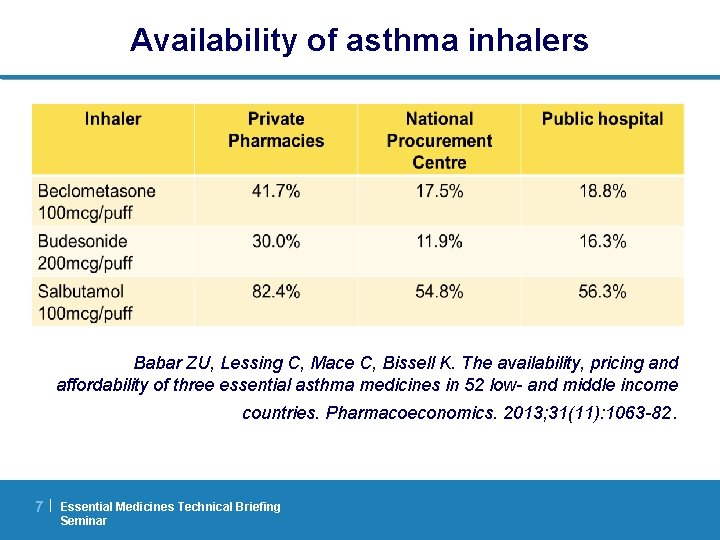
Prices of insulin per 10 ml 100 IU vial Beran D, Yudkin JS. Looking beyond the issue of access to insulin: what is needed for proper diabetes care in resource poor settings. Diabetes Res Clin Pract. 2010; 88(3): 217 -21 8| Essential Medicines Technical Briefing Seminar

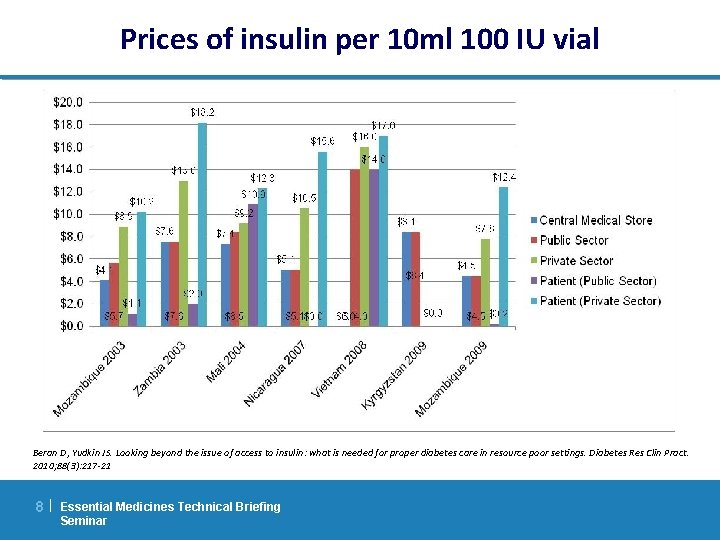
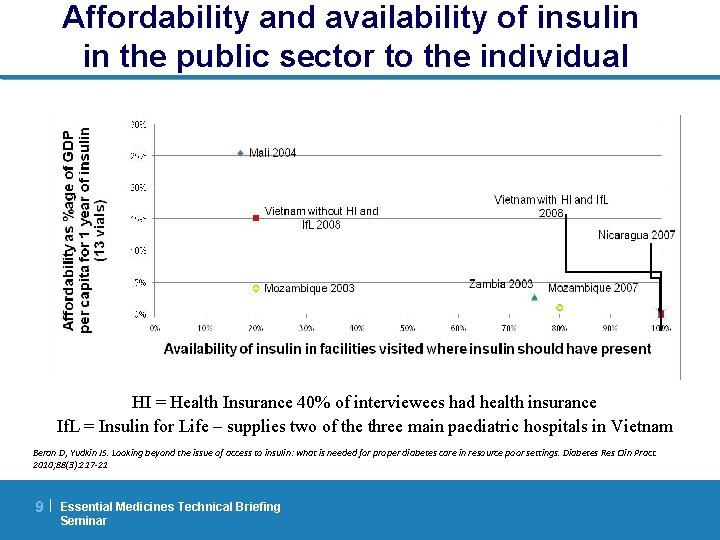
Affordability and availability of insulin in the public sector to the individual HI = Health Insurance 40% of interviewees had health insurance If. L = Insulin for Life – supplies two of the three main paediatric hospitals in Vietnam Beran D, Yudkin JS. Looking beyond the issue of access to insulin: what is needed for proper diabetes care in resource poor settings. Diabetes Res Clin Pract. 2010; 88(3): 217 -21 9| Essential Medicines Technical Briefing Seminar

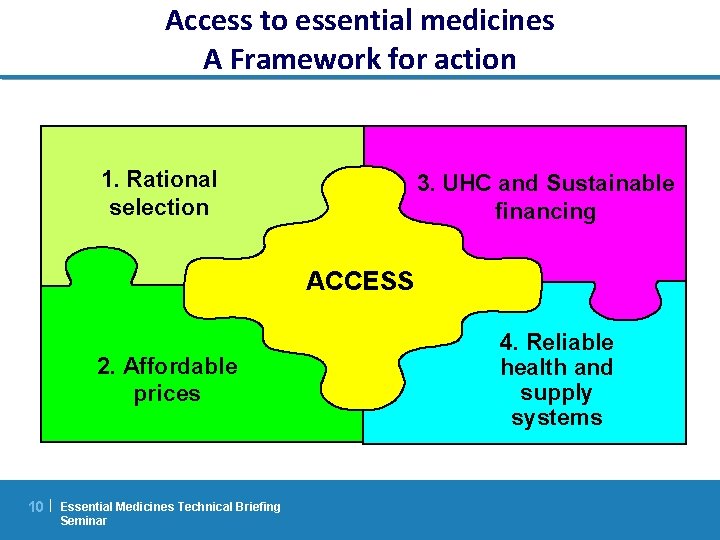
Access to essential medicines A Framework for action 1. Rational selection 3. UHC and Sustainable financing ACCESS 2. Affordable prices 10 | Essential Medicines Technical Briefing Seminar 4. Reliable health and supply systems

Options to improve the situation l Rational selection – Standard Therapeutic Guidelines including evidence-based selection of medicines (NEML based on recommendations from WHO Expert Committee on Selection and Rational Use and PEN guidelines) – Alignment of NEML and STGs as a basis for procurement, reimbursement and training of staff – Promotion of the use of STGs and NEML by health care professionals and proper training of staff on STGs l Financing and Universal Health Coverage – Increase government budget to ensure widespread access to a reduced number of NCD essential medicines – Expand coverage/health insurance and ensure NCD essential medicines are part of the essential package covered 11 | Essential Medicines Technical Briefing Seminar

Options to improve the situation l Price – Competition, promotion of generics, transparent procurement procedures, substitution, reduce duties/taxes and mark-ups, monitoring prices – For single source products or under patent, apply WTO/TRIPS flexibilities and use available differential pricing l Reliable Supply systems – Supply quality-assured products: reinforce NRAs and limit SSFFC products in the supply chain, increase confidence of prescribers in generic products – Improve quantification of needs and forecasting using good information systems – Reinforce private and public supply chains 12 | Essential Medicines Technical Briefing Seminar

Perspective and Role for WHO/EMP l Sensitize countries and partners to the barriers for access to essential medicines and health technologies for NCDs l Develop and promote monitoring tools to document the situation, to identify priority interventions and measure improvements in access over time l Update WHO Model List of Essential Medicines to address NCD issues (e. g. cancer section) l Update and promote use of National Standard Therapeutic guidelines l Continue the country support to strengthen NRAs and supply systems and develop relevant policies (including pricing policies) l Support global and regional initiatives for information sharing on medicines prices and availability 13 | Essential Medicines Technical Briefing Seminar

Recent Global Initiatives l Global Coordination Mechanism – Working Groups on NCDs – Active private sector participation – Innovative financing for NCDs l UN Inter Agency Task Force on NCDs – 42 working teams – access to essential medicines for NCDs being one of them l WHO working plan – Discussion Paper – Action points for WHO and Member States on NCD medicines – To be peer-reviewed and posted on website for public comments before the end of the year 14 | Essential Medicines Technical Briefing Seminar

THANK YOU
 Plamatic acid
Plamatic acid Abasagar
Abasagar The drug basket method used to dispense medication to
The drug basket method used to dispense medication to Pharmac pharmaceutical schedule
Pharmac pharmaceutical schedule Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Chapter 19 lesson 2 using medicines safely
Chapter 19 lesson 2 using medicines safely Medicines complete
Medicines complete 10 herbal plants approved by doh
10 herbal plants approved by doh National medicines policy
National medicines policy Stockley’s drug interaction
Stockley’s drug interaction George's marvellous medicine story
George's marvellous medicine story Medicines information centre
Medicines information centre Cqc medicines management
Cqc medicines management European directorate for the quality of medicines
European directorate for the quality of medicines Ggc medicines
Ggc medicines Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines