9 th IAS Conference on HIV Science IAS










- Slides: 11

9 th IAS Conference on HIV Science (IAS 2017) NIH Grant mechanisms: K, R, P, U Roberta Binder, Ph. D Scientific Review Officer Scientific Review Program Division of Extramural Activities rbinder@niaid. nih. gov No conflicts of interest to declare July 26, 2017

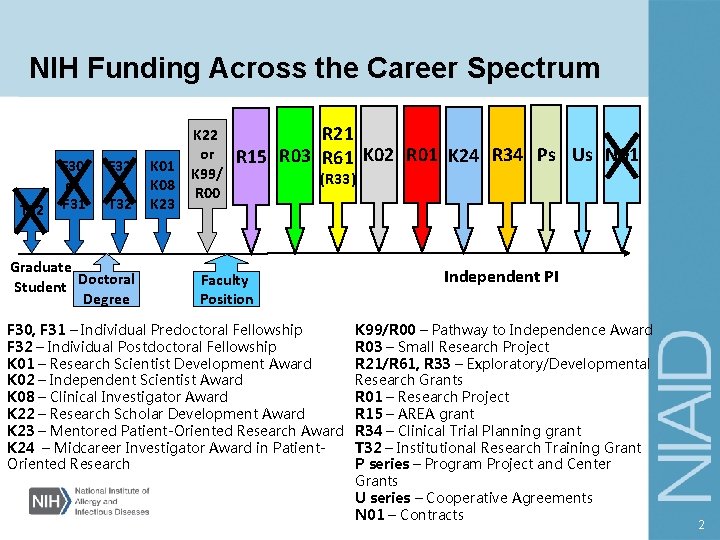
NIH Funding Across the Career Spectrum T 32 F 30 or F 31 F 32 or T 32 Graduate Student Doctoral Degree K 01 K 08 K 23 K 22 or K 99/ R 00 R 21 R 15 R 03 R 61 K 02 R 01 K 24 R 34 Ps Us N 01 (R 33) Faculty Position F 30, F 31 – Individual Predoctoral Fellowship F 32 – Individual Postdoctoral Fellowship K 01 – Research Scientist Development Award K 02 – Independent Scientist Award K 08 – Clinical Investigator Award K 22 – Research Scholar Development Award K 23 – Mentored Patient-Oriented Research Award K 24 – Midcareer Investigator Award in Patient. Oriented Research Independent PI K 99/R 00 – Pathway to Independence Award R 03 – Small Research Project R 21/R 61, R 33 – Exploratory/Developmental Research Grants R 01 – Research Project R 15 – AREA grant R 34 – Clinical Trial Planning grant T 32 – Institutional Research Training Grant P series – Program Project and Center Grants U series – Cooperative Agreements N 01 – Contracts 2

Mentored Career Development (K) Awards q K 01—Mentored Research Scientist Development Award § Research in biomedical, behavioral or clinical science § Candidate must have a research or health-professional doctoral degree q K 08—Mentored Clinical Scientist Research Career Development Award § Research in biomedical, behavioral areas including translational science § Candidate must have a clinical doctoral (e. g. MD, DVM) degree q K 23—Mentored Patient-Oriented Research Career Development Award § Supports patient-oriented research § Candidate must have a health professional doctoral degree 3

Mentored Career Development (K) Awards q K 25—Mentored Quantitative Research Development Award § Supports the integration of quantitative or engineering investigators with NIH-relevant health and disease research § Candidate must have an advanced degree in quantitative area of science or engineering Ø Support (up to 5 years) career development experiences for attaining research independence Ø Only US Citizens, Non-citizen Nationals, or Permanent Residents Ø NIH ICs may have additional eligibility requirements; Contact a Program Officer in your subject area before applying 4

Career Development (K) Awards q K 02—Independent Scientist Award (up to 5 years) • Supports newly independent investigators • Candidate must have independent, peer-reviewed research support Ø Only US Citizens, Non-citizen Nationals, or Permanent Residents q K 22— Career Transition Award (up to 2 years) • Supports transition from a post-doctoral fellow to an independent investigator position • Candidate must secure a full time tenure-track position for the release of the award Ø Only US Citizens, Non-citizen Nationals, or Permanent Residents q K 24—Midcareer Investigator Award in Patient-Oriented Research (up to 5 years) • For mid-career investigators to mentor clinical residents/fellows/junior faculty • Candidate must have independent, peer-reviewed patient-oriented research support Ø Only US Citizens, Non-citizen Nationals, or Permanent Residents q K 99/R 00—NIH Pathway to Independence Award (2 yrs. K 99 + 3 yrs. R 00) • Supports transition from a mentored post-doc to an independent faculty position • Candidate CANNOT have more than 4 years of postdoctoral research experience Ø Non-US citizens with temporary US visas are eligible to apply 5

NIH Research Grant Programs (R Series) q § § R 03—Small Grant Program (up to 2 years; $50 K/year) Supports small research projects requiring limited resources Appropriate for pilot and feasibility studies, research methodology/technology development NO preliminary data requirement; Award is non-renewable Non-US Institutions are eligible to apply q § § R 21—Exploratory/Developmental Research Grant Program (up to 2 years, $275 K) Support for early and conceptual stages of exploratory and developmental research projects Foster novel scientific ideas, tools, and technologies NO preliminary data requirement; Award is non-renewable Non-US Institutions are eligible to apply Ø The R 61 award is used when larger budgets and/or project periods are required to establish feasibility for the project Ø The R 33 award is the second phase for the support of research activities initiated under the R 21 or R 61 mechanism q § § R 01—Research Project Grant Program (up to 5 years; no set budget limit) NIH’s most commonly used grant program Supports discrete, specified, circumscribed research project Preliminary data is encouraged; Possibility to renew through the NIH peer review process Non-US Institutions are eligible to apply 6

NIH Research Grant Programs (R Series) q R 15—NIH Academic Research Enhancement Award (AREA) (up to 3 years , $300 K) § Support investigators at small four-year colleges and universities that train significant numbers of research undergraduates but have a limited share of NIH funds § Small scale research projects to expose students to research and to strengthen applicant institution’s research environment § NO preliminary data requirement; Possibility to renew through the NIH peer review process § Only US-based Institutions are eligible to apply q R 34—NIH Clinical Trial Planning Grant Program (up to 3 years; budget limit as per FOA) § Permit early peer review of the scientific rationale and concept for the proposed clinical trial § Support development of essential elements of the trial § Award is non-renewable § Non-US Institutions’ eligibility depends on FOA q R 24—Resource-Related Research Project (up to 5 years; budget limit as per FOA) § Develop specialized resources that can be of significant benefit to existing research programs (e. g. data curation tools for a multi-center epidemiological study) § Contact NIH staff to discuss your idea before applying; Used by a limited number of NIH ICs § Only US-based Institutions are eligible to apply 7

NIH Program Project/Center Grants (P Series) q. P 01—Research Program Project Grant • Supports an integrated, multi-directional, Research Program involving several independent investigators who share knowledge and common resources • Includes two or more research projects, administrative core, and scientific cores that provide services to research projects; e. g. HIV Vaccine Research and Design (HIVRAD) q. P 30—Center Core Grant • Support scientific/technical research cores for a pool of investigators undertaking a multidisciplinary approach to a research problem • Foster synergy, improve research coordination, and promote efficient use of resources shared by multiple independent laboratories; e. g. NIH Centers for AIDS Research (CFAR) 8

NIH Program Project/Center Grants (P Series) q. P 50—Specialized Center • Support a full range of R&D activities for a multidisciplinary approach to a specific research problem • Can include activities from very basic research to clinical development • Centers may serve as regional or national resources for special research purposes; e. g. Alzheimer’s Disease Research Centers ØGenerally led by an established investigator(s) with new investigators as Project/Core Leaders ØPreliminary data is expected; Synergy in the research effort is emphasized ØEligibility of non-US Institutions, duration of support and budget limit is specified in the FOA 9

Cooperative Agreements (U Series) q. U 01— Research Project Cooperative Agreement • Discrete, specified, circumscribed projects to be performed by investigator(s) in an area representing their specific interests and competencies q. UM 1— Research Project with Complex Structure Cooperative Agreement • Large-scale research activities with complicated structures that cannot be appropriately categorized into an available single component activity code, e. g. clinical networks, research programs or consortium q. UM 2— Program Project or Center with Complex Structure Cooperative Agreement • Program projects or centers with complicated structures that cannot be appropriately categorized into an available multicomponent activity code, e. g. clinical networks, research programs or consortium q. U 19 — Research Program Cooperative Agreement • Research Programs with a combination of Research Projects and Cores; e. g. Integrated Preclinical/Clinical AIDS Vaccine Development (IPCAVD) Program ØSubstantial NIH Program staff involvement to assist PI during performance of the research activities, as defined in the terms and conditions of the award ØPI responsible for planning, directing, and executing the project ØEligibility of non-US Institutions, duration of support/budget as per the FOA 10

Resources § NIH Grants & Funding website: • https: //grants. nih. gov/grants/oer. htm § Comprehensive list of all Activity Codes: • https: //grants. nih. gov/grants/funding/ac_search_res ults. htm 11
 Hiv diagnostics conference
Hiv diagnostics conference Ias-17 conference
Ias-17 conference What is your favorite subject
What is your favorite subject Wind energy science conference
Wind energy science conference Atlanta conference on science and innovation policy
Atlanta conference on science and innovation policy Informs marketing science
Informs marketing science Triệu chứng nhiễm hiv
Triệu chứng nhiễm hiv Hiv reverse transcription
Hiv reverse transcription Test wiedzy o hiv i aids z odpowiedziami
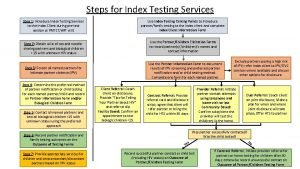
Test wiedzy o hiv i aids z odpowiedziami 10 steps of index testing
10 steps of index testing Prognas snars edisi 1.1
Prognas snars edisi 1.1 Phdp in hiv
Phdp in hiv