8020 THINK What do you NEED to cover



- Slides: 16

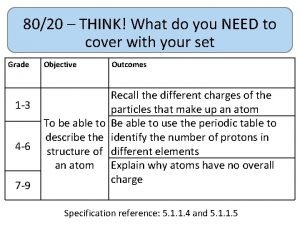
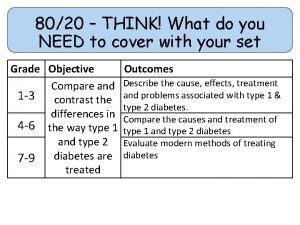
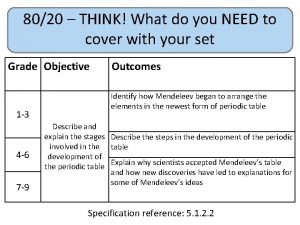
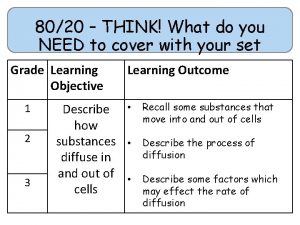
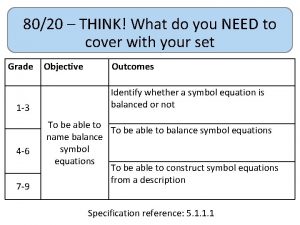
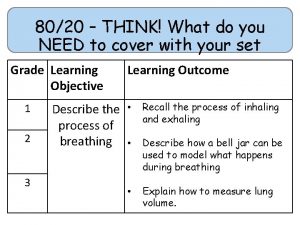
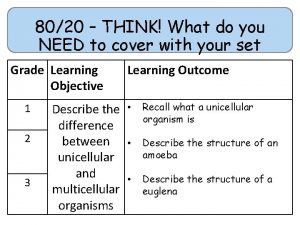
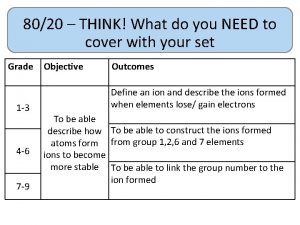
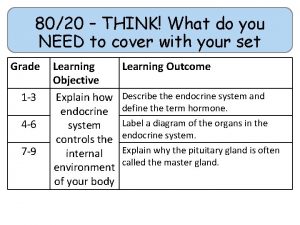
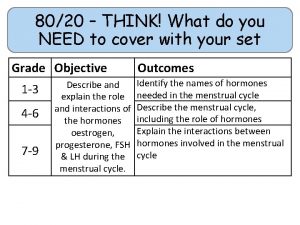
80/20 – THINK! What do you NEED to cover with your set Grade 1 -3 4 -6 7 -9 Objective Outcomes Use given data to describe the trends as you To be able to go down group 7 describe and predict the Explain how properties of the elements in properties and Group 7 depend on the outer shell of reactions of electrons of the atoms and predict elements properties from given trends down the found in Group group 7 based upon Construct balanced symbol equations for their electronic displacement reactions of halogens configuration Specification reference: 5. 1. 2. 6

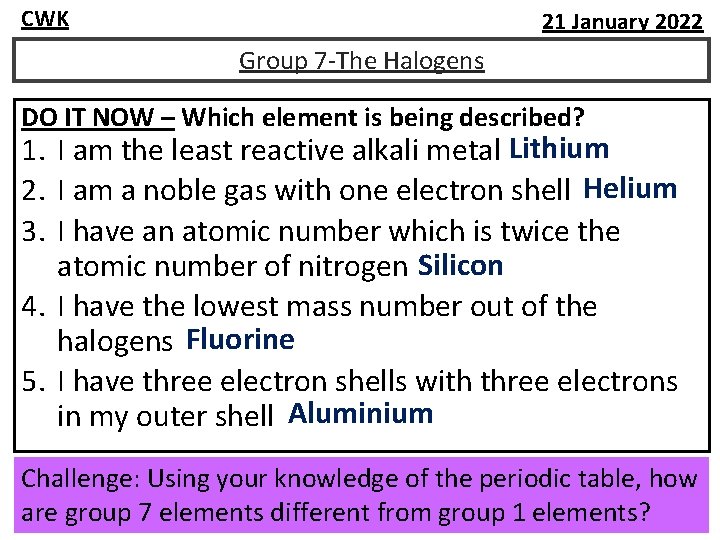
CWK 21 January 2022 Group 7 -The Halogens DO IT NOW – Which element is being described? 1. I am the least reactive alkali metal Lithium 2. I am a noble gas with one electron shell Helium 3. I have an atomic number which is twice the atomic number of nitrogen Silicon 4. I have the lowest mass number out of the halogens Fluorine 5. I have three electron shells with three electrons in my outer shell Aluminium Challenge: Using your knowledge of the periodic table, how are group 7 elements different from group 1 elements?

Progress indicators GOOD PROGRESS: OUTSTANDING PROGRESS:

Word consciousness Displacement – when a more reactive element pushes a less reactive element out of a compound

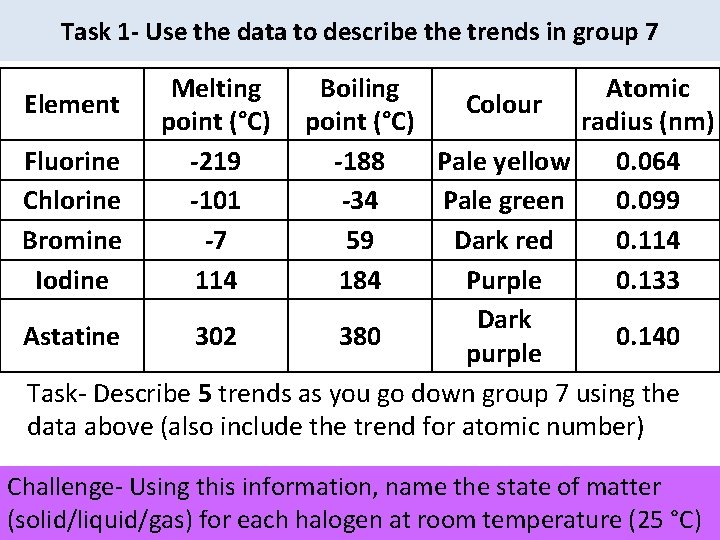
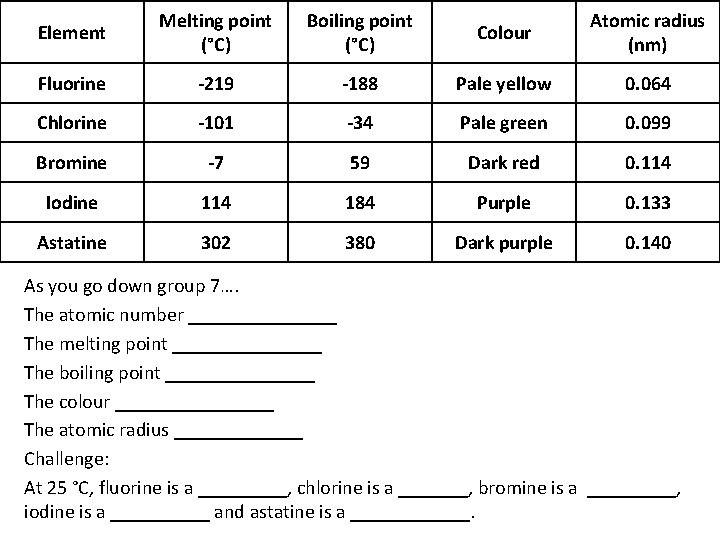
Task 1 - Use the data to describe the trends in group 7 Melting point (°C) -219 -101 -7 114 Boiling Atomic Element Colour point (°C) radius (nm) Fluorine -188 Pale yellow 0. 064 Chlorine -34 Pale green 0. 099 Bromine 59 Dark red 0. 114 Iodine 184 Purple 0. 133 Dark Astatine 302 380 0. 140 purple Task- Describe 5 trends as you go down group 7 using the data above (also include the trend for atomic number) Challenge- Using this information, name the state of matter (solid/liquid/gas) for each halogen at room temperature (25 °C)

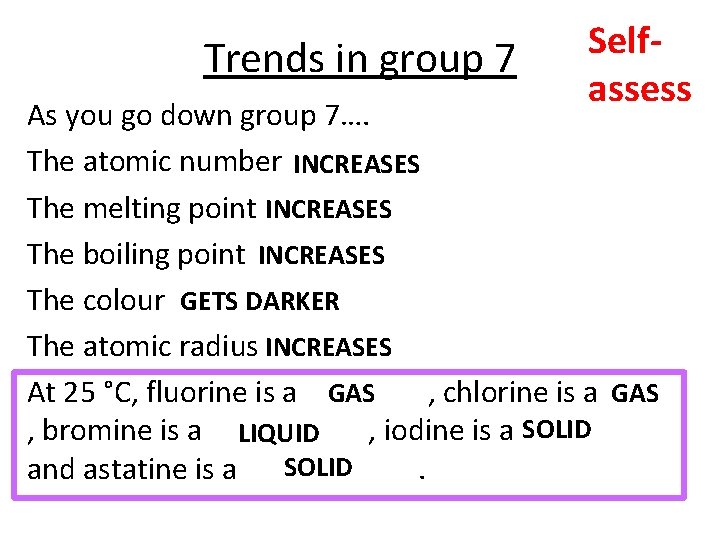
Trends in group 7 Selfassess As you go down group 7…. The atomic number INCREASES The melting point INCREASES The boiling point INCREASES The colour GETS DARKER The atomic radius INCREASES At 25 °C, fluorine is a GAS , chlorine is a GAS , bromine is a LIQUID , iodine is a SOLID and astatine is a SOLID.

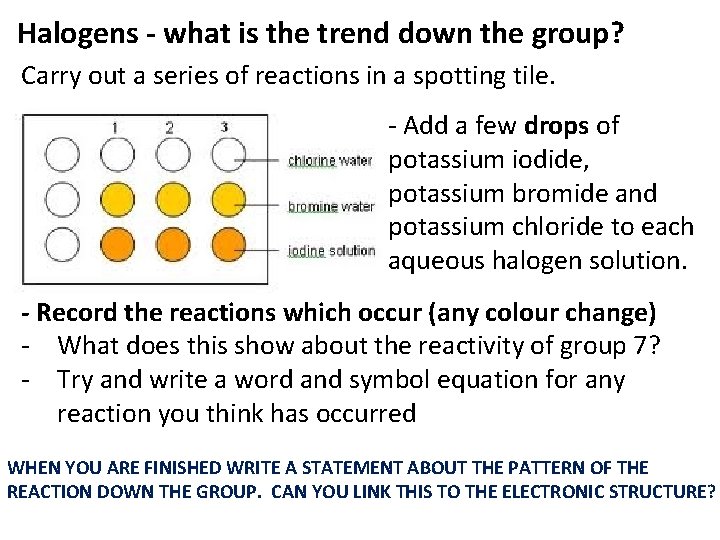
Halogens - what is the trend down the group? Carry out a series of reactions in a spotting tile. - Add a few drops of potassium iodide, potassium bromide and potassium chloride to each aqueous halogen solution. - Record the reactions which occur (any colour change) - What does this show about the reactivity of group 7? - Try and write a word and symbol equation for any reaction you think has occurred WHEN YOU ARE FINISHED WRITE A STATEMENT ABOUT THE PATTERN OF THE REACTION DOWN THE GROUP. CAN YOU LINK THIS TO THE ELECTRONIC STRUCTURE?

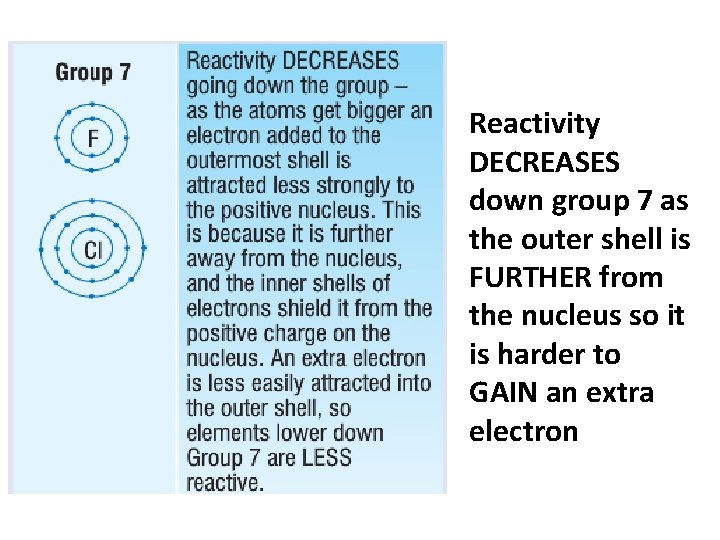
Reactivity DECREASES down group 7 as the outer shell is FURTHER from the nucleus so it is harder to GAIN an extra electron

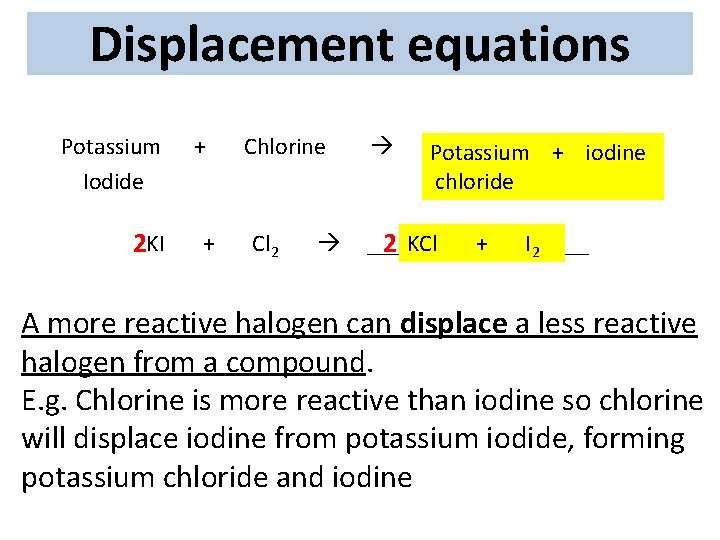
Displacement equations Potassium Iodide 2 KI + + Chlorine Cl 2 _______ + + ______ Potassium iodine _______ chloride _______ 2 KCl ++ ______ I 2 A more reactive halogen can displace a less reactive F halogen from a compound. Cl E. g. Chlorine is more reactive than iodine so chlorine Br potassium iodide, forming will displace iodine from potassium chloride and. Iiodine

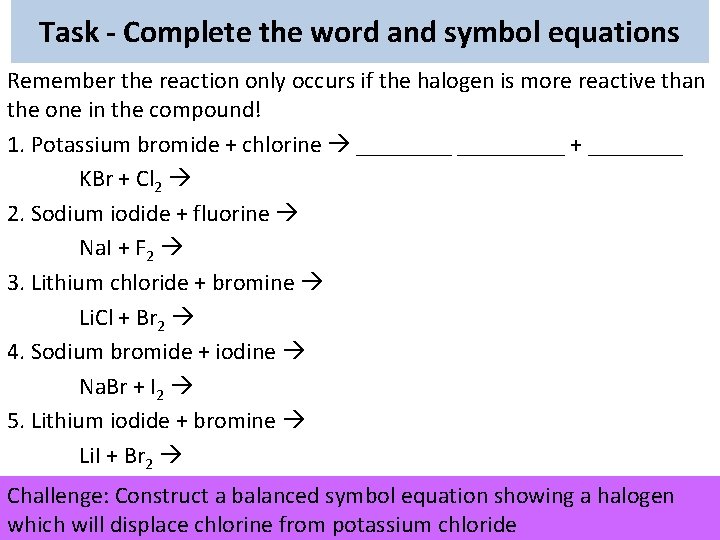
Task - Complete the word and symbol equations Remember the reaction only occurs if the halogen is more reactive than the one in the compound! 1. Potassium bromide + chlorine _________ + ____ KBr + Cl 2 2. Sodium iodide + fluorine Na. I + F 2 3. Lithium chloride + bromine Li. Cl + Br 2 4. Sodium bromide + iodine Na. Br + I 2 5. Lithium iodide + bromine Li. I + Br 2 Challenge: Construct a balanced symbol equation showing a halogen which will displace chlorine from potassium chloride

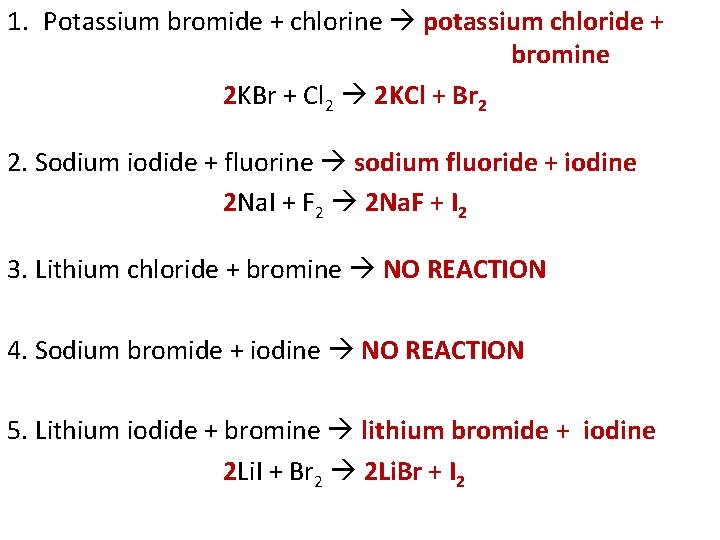
1. Potassium bromide + chlorine potassium chloride + bromine 2 KBr + Cl 2 2 KCl + Br 2 2. Sodium iodide + fluorine sodium fluoride + iodine 2 Na. I + F 2 2 Na. F + I 2 3. Lithium chloride + bromine NO REACTION 4. Sodium bromide + iodine NO REACTION 5. Lithium iodide + bromine lithium bromide + iodine 2 Li. I + Br 2 2 Li. Br + I 2

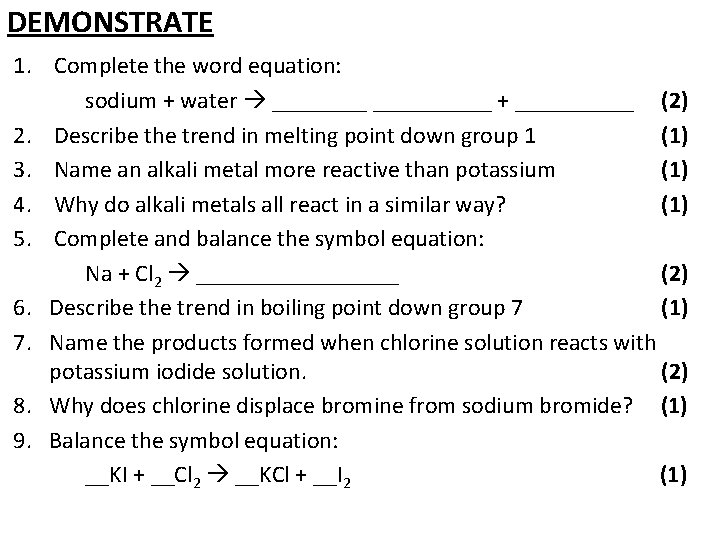
DEMONSTRATE 1. Complete the word equation: sodium + water __________ + _____ (2) 2. Describe the trend in melting point down group 1 (1) 3. Name an alkali metal more reactive than potassium (1) 4. Why do alkali metals all react in a similar way? (1) 5. Complete and balance the symbol equation: Na + Cl 2 _________ (2) 6. Describe the trend in boiling point down group 7 (1) 7. Name the products formed when chlorine solution reacts with potassium iodide solution. (2) 8. Why does chlorine displace bromine from sodium bromide? (1) 9. Balance the symbol equation: __KI + __Cl 2 __KCl + __I 2 (1)

1. Complete the word equation: sodium + water sodium hydroxide + hydrogen 2. Describe the trend in melting point down group 1 (2) (1) As you go down group 1, the melting point decreases 3. Name an alkali metal more reactive than potassium (1) One of: Rubidium, caesium or francium 4. Why do alkali metals all react in a similar way? (1) They all have one electron in their outer shell 5. Complete and balance the symbol equation: 2 Na + Cl 2 2 Na. Cl One mark for Na. Cl formula, one mark for correct balancing (2)

6. Describe the trend in boiling point down group 7 (1) As you go down group 7, the boiling point increases 7. Name the products formed when chlorine solution reacts with potassium iodide solution. (2) - Potassium chloride - Iodine 8. Why does chlorine displace bromine from sodium bromide? (1) Chlorine is more reactive than bromine 9. Balance the symbol equation: 2 KI + __Cl 2 2 KCl + __I 2 (1)

Plenary Name: • 3 halogens which are more reactive than astatine • 2 products from a reaction between potassium bromide and fluorine • 1 reason fluorine is more reactive than chlorine

Element Melting point (°C) Boiling point (°C) Colour Atomic radius (nm) Fluorine -219 -188 Pale yellow 0. 064 Chlorine -101 -34 Pale green 0. 099 Bromine -7 59 Dark red 0. 114 Iodine 114 184 Purple 0. 133 Astatine 302 380 Dark purple 0. 140 As you go down group 7…. The atomic number ________ The melting point ________ The boiling point ________ The colour ________ The atomic radius _______ Challenge: At 25 °C, fluorine is a _____, chlorine is a _______, bromine is a _____, iodine is a _____ and astatine is a ______.
 Iso 8020
Iso 8020 Ramaniklal ambani
Ramaniklal ambani If you think you can you can poem
If you think you can you can poem Latent strabismus
Latent strabismus Intermittent exotropia
Intermittent exotropia Harmonious arc
Harmonious arc I wish you strenght
I wish you strenght Speech
Speech Think said the robin
Think said the robin Think fam think
Think fam think Have a daughter so you can argue
Have a daughter so you can argue Choose the correct answers a-c how do you think you
Choose the correct answers a-c how do you think you So you think you can argue
So you think you can argue Minecraft did you know
Minecraft did you know What comes to your mind when you hear the word family
What comes to your mind when you hear the word family You have more potential than you think
You have more potential than you think When you hear the word scientist what comes to mind
When you hear the word scientist what comes to mind