8020 THINK What do you NEED to cover



- Slides: 19

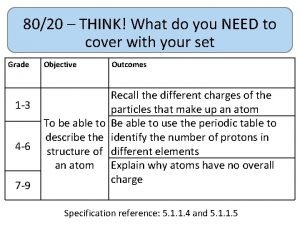
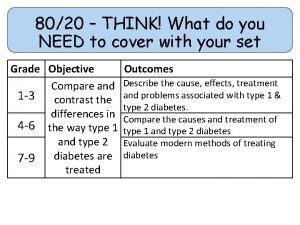
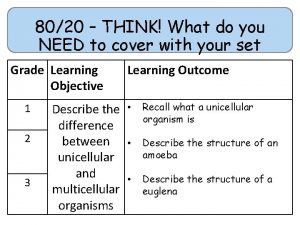
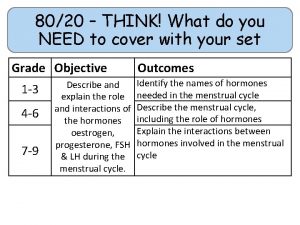
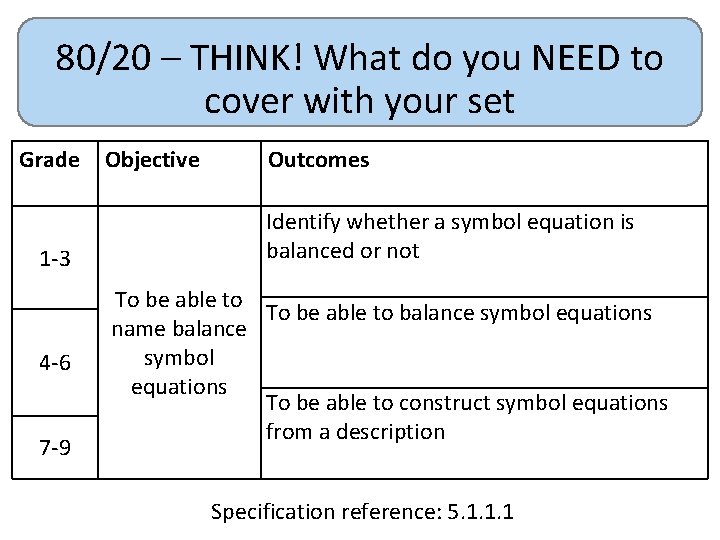
80/20 – THINK! What do you NEED to cover with your set Grade 1 -3 4 -6 7 -9 Objective Outcomes Identify whether a symbol equation is balanced or not To be able to balance symbol equations name balance symbol equations To be able to construct symbol equations from a description Specification reference: 5. 1. 1. 1

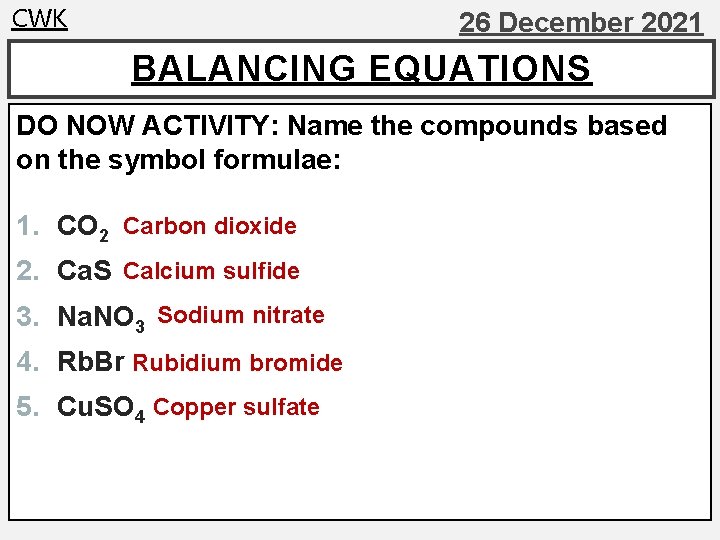
CWK 26 December 2021 BALANCING EQUATIONS DO NOW ACTIVITY: Name the compounds based on the symbol formulae: 1. CO 2 Carbon dioxide 2. Ca. S Calcium sulfide 3. Na. NO 3 Sodium nitrate 4. Rb. Br Rubidium bromide 5. Cu. SO 4 Copper sulfate

Progress indicators GOOD PROGRESS: OUTSTANDING PROGRESS:

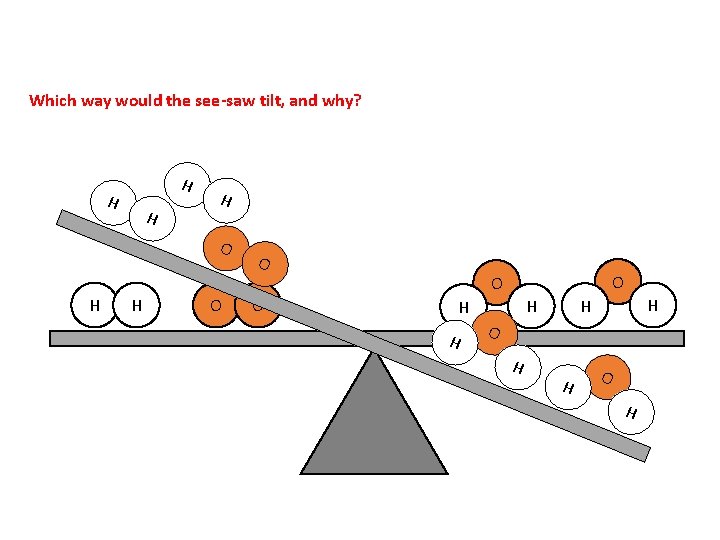
Word consciousness Conservation – remains the same (e. g. mass in a reaction)

Which way would the see-saw tilt, and why? H H O O O H H H O H

Conservation of Mass is neither created nor destroyed during chemical processes. It is conserved. Antoine Lavoisier – Old School Chemist

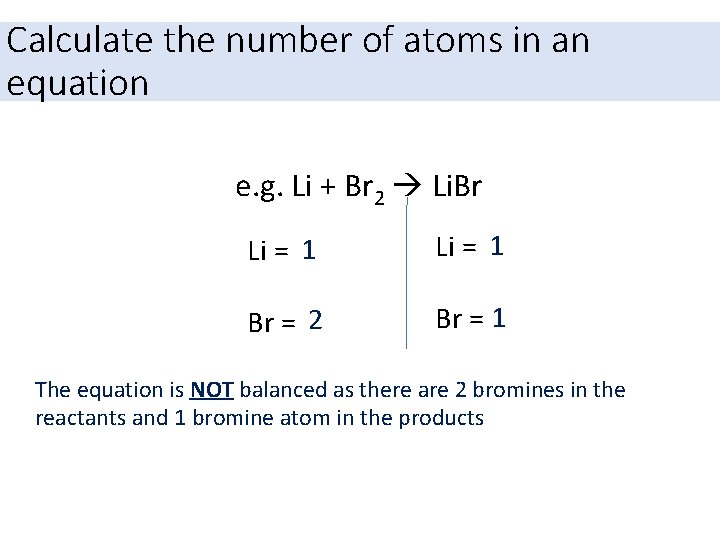
Calculate the number of atoms in an equation e. g. Li + Br 2 Li. Br Li = 1 Br = 2 Br = 1 The equation is NOT balanced as there are 2 bromines in the reactants and 1 bromine atom in the products

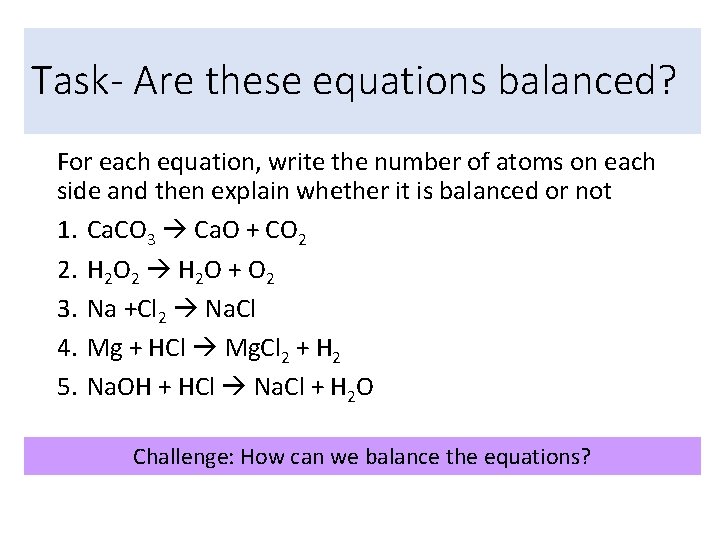
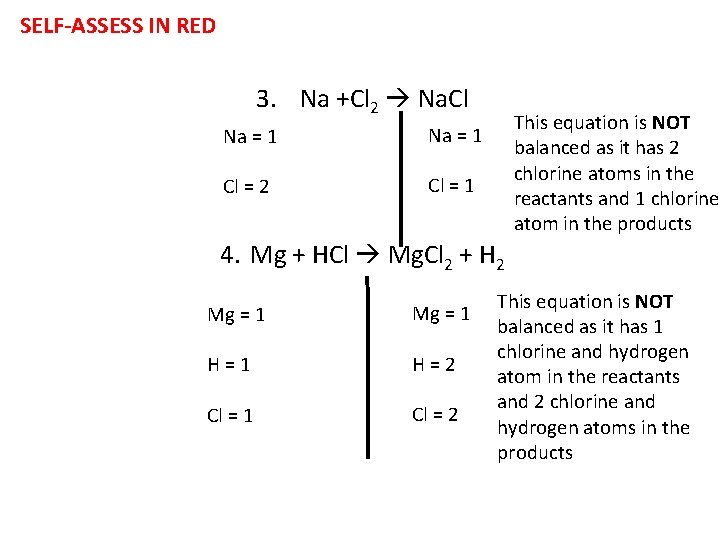
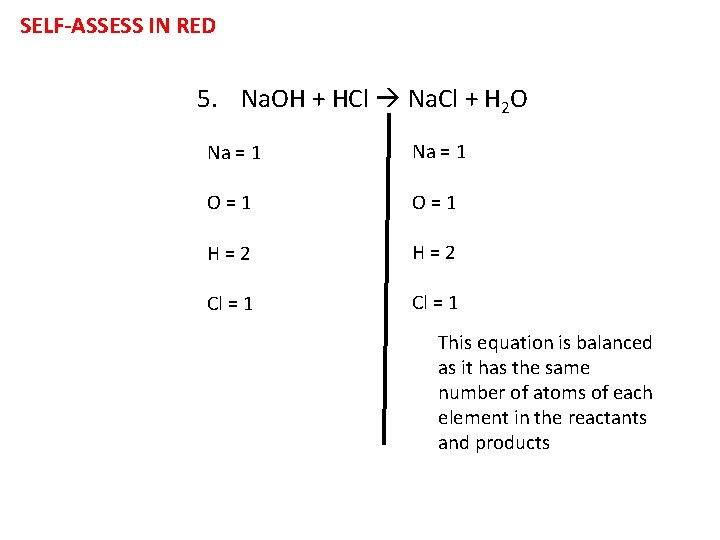
Task- Are these equations balanced? For each equation, write the number of atoms on each side and then explain whether it is balanced or not 1. Ca. CO 3 Ca. O + CO 2 2. H 2 O 2 H 2 O + O 2 3. Na +Cl 2 Na. Cl 4. Mg + HCl Mg. Cl 2 + H 2 5. Na. OH + HCl Na. Cl + H 2 O Challenge: How can we balance the equations?

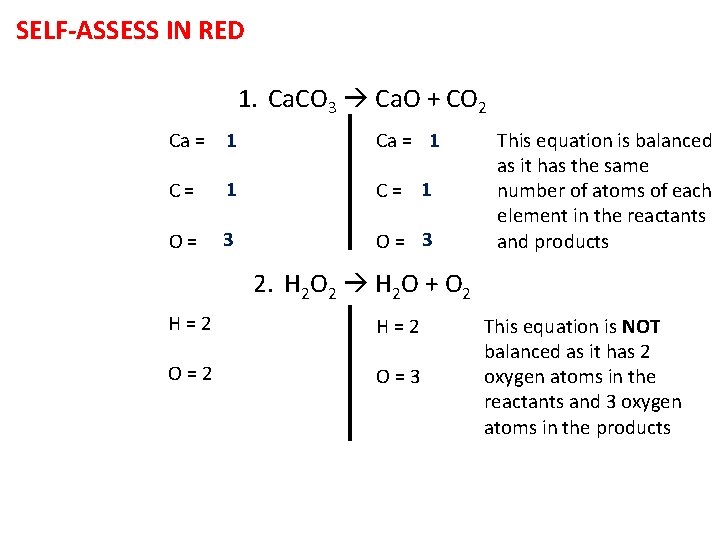
SELF-ASSESS IN RED 1. Ca. CO 3 Ca. O + CO 2 Ca = 1 C= 1 O= 3 This equation is balanced as it has the same number of atoms of each element in the reactants and products 2. H 2 O 2 H 2 O + O 2 H=2 O=3 This equation is NOT balanced as it has 2 oxygen atoms in the reactants and 3 oxygen atoms in the products

SELF-ASSESS IN RED 3. Na +Cl 2 Na. Cl Na = 1 Cl = 2 Cl = 1 This equation is NOT balanced as it has 2 chlorine atoms in the reactants and 1 chlorine atom in the products 4. Mg + HCl Mg. Cl 2 + H 2 Mg = 1 H=1 H=2 Cl = 1 Cl = 2 This equation is NOT balanced as it has 1 chlorine and hydrogen atom in the reactants and 2 chlorine and hydrogen atoms in the products

SELF-ASSESS IN RED 5. Na. OH + HCl Na. Cl + H 2 O Na = 1 O=1 H=2 Cl = 1 This equation is balanced as it has the same number of atoms of each element in the reactants and products

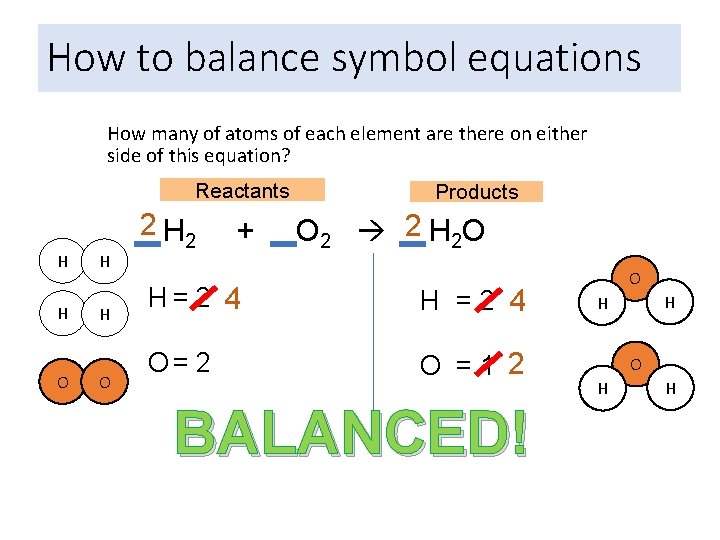
How to balance symbol equations How many of atoms of each element are there on either side of this equation? Reactants H H O O 2 H 2 + Products O 2 2 H 2 O H= 2 4 H =2 4 O= 2 O =1 2 BALANCED! O H H

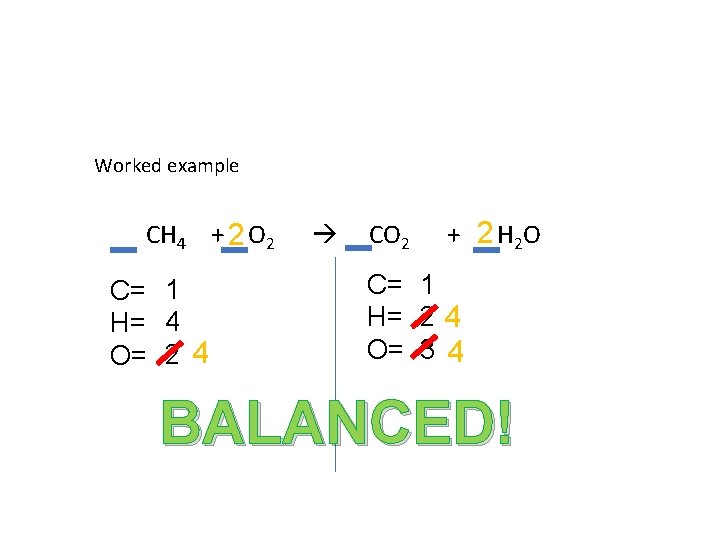
Worked example CH 4 + 2 O 2 C= 1 H= 4 O= 2 4 CO 2 + 2 H 2 O C= 1 H= 2 4 O= 3 4 BALANCED!


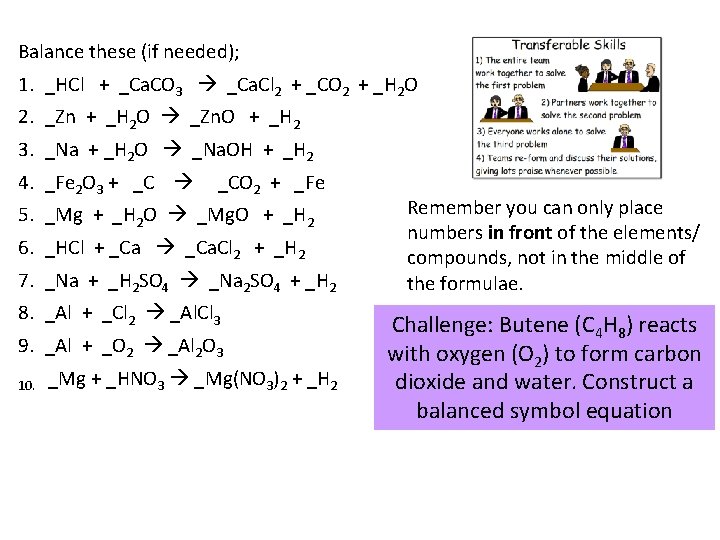
Balance these (if needed); 1. _HCl + _Ca. CO 3 _Ca. Cl 2 + _CO 2 + _H 2 O 2. _Zn + _H 2 O _Zn. O + _H 2 3. _Na + _H 2 O _Na. OH + _H 2 4. _Fe 2 O 3 + _C _CO 2 + _Fe 5. _Mg + _H 2 O _Mg. O + _H 2 6. _HCl + _Ca. Cl 2 + _H 2 7. _Na + _H 2 SO 4 _Na 2 SO 4 + _H 2 8. _Al + _Cl 2 _Al. Cl 3 9. _Al + _O 2 _Al 2 O 3 10. _Mg + _HNO 3 _Mg(NO 3)2 + _H 2 Remember you can only place numbers in front of the elements/ compounds, not in the middle of the formulae. Challenge: Butene (C 4 H 8) reacts with oxygen (O 2) to form carbon dioxide and water. Construct a balanced symbol equation

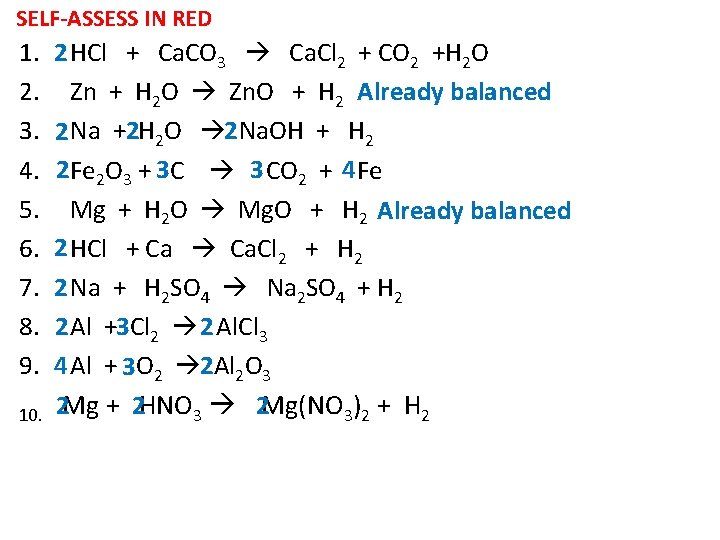
SELF-ASSESS IN RED 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 2 HCl + Ca. CO 3 Ca. Cl 2 + CO 2 +H 2 O Zn + H 2 O Zn. O + H 2 Already balanced 2 Na +2 H 2 O 2 Na. OH + H 2 2 Fe 2 O 3 + 3 C 3 CO 2 + 4 Fe Mg + H 2 O Mg. O + H 2 Already balanced 2 HCl + Ca Ca. Cl 2 + H 2 2 Na + H 2 SO 4 Na 2 SO 4 + H 2 2 Al +3 Cl 2 2 Al. Cl 3 4 Al + 3 O 2 2 Al 2 O 3 2 Mg + 2 HNO 3 2 Mg(NO 3)2 + H 2

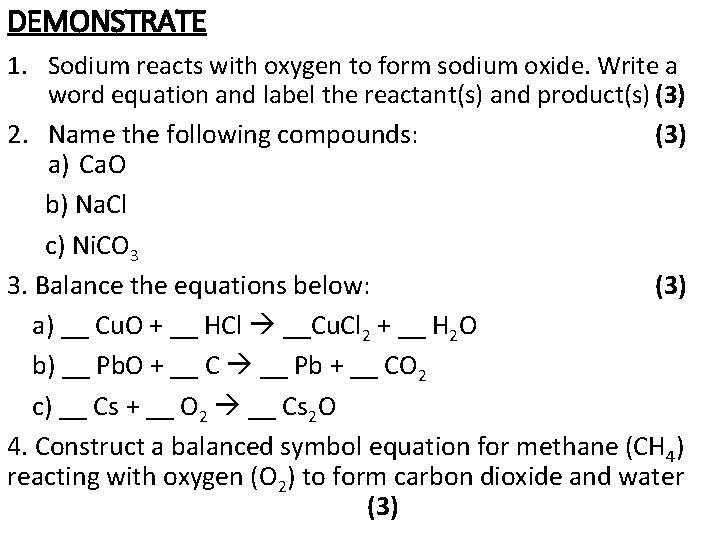
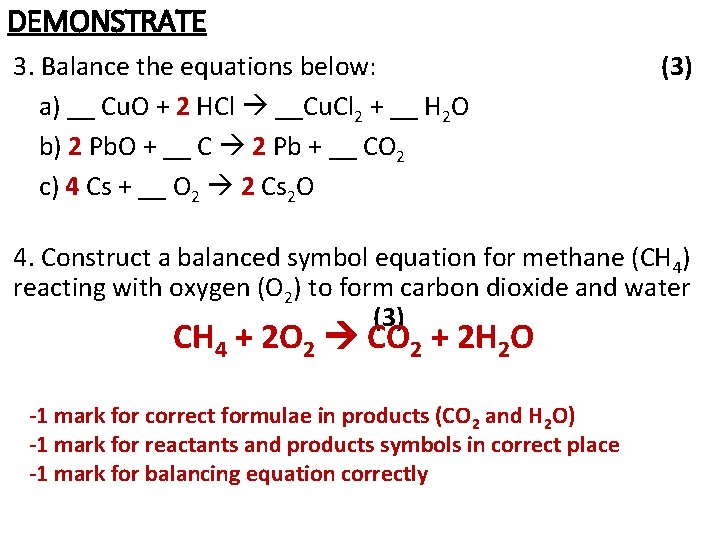
DEMONSTRATE 1. Sodium reacts with oxygen to form sodium oxide. Write a word equation and label the reactant(s) and product(s) (3) 2. Name the following compounds: (3) a) Ca. O b) Na. Cl c) Ni. CO 3 3. Balance the equations below: (3) a) __ Cu. O + __ HCl __Cu. Cl 2 + __ H 2 O b) __ Pb. O + __ C __ Pb + __ CO 2 c) __ Cs + __ O 2 __ Cs 2 O 4. Construct a balanced symbol equation for methane (CH 4) reacting with oxygen (O 2) to form carbon dioxide and water (3)

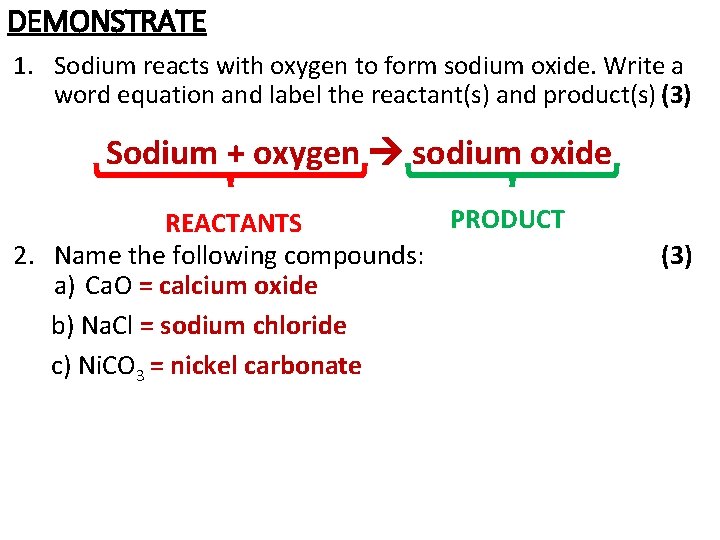
DEMONSTRATE 1. Sodium reacts with oxygen to form sodium oxide. Write a word equation and label the reactant(s) and product(s) (3) Sodium + oxygen sodium oxide PRODUCT REACTANTS 2. Name the following compounds: a) Ca. O = calcium oxide b) Na. Cl = sodium chloride c) Ni. CO 3 = nickel carbonate (3)

DEMONSTRATE 3. Balance the equations below: a) __ Cu. O + 2 HCl __Cu. Cl 2 + __ H 2 O b) 2 Pb. O + __ C 2 Pb + __ CO 2 c) 4 Cs + __ O 2 2 Cs 2 O (3) 4. Construct a balanced symbol equation for methane (CH 4) reacting with oxygen (O 2) to form carbon dioxide and water (3) CH 4 + 2 O 2 CO 2 + 2 H 2 O -1 mark for correct formulae in products (CO 2 and H 2 O) -1 mark for reactants and products symbols in correct place -1 mark for balancing equation correctly
 Dvx-9000
Dvx-9000 Think big think fast
Think big think fast If you think you can you can poem
If you think you can you can poem Manifest squint meaning
Manifest squint meaning Alternate prism cover test
Alternate prism cover test Worth 4 dot test
Worth 4 dot test I wish you strength
I wish you strength New speaker new line worksheet
New speaker new line worksheet A little bird by aileen fisher
A little bird by aileen fisher Think fam think
Think fam think Have a daughter so you can argue
Have a daughter so you can argue Choose the correct answers ac
Choose the correct answers ac So you think you can argue
So you think you can argue You are
You are What comes to mind when you hear the word 'family'?
What comes to mind when you hear the word 'family'? You have more potential than you think
You have more potential than you think When you hear the word scientist what comes to mind
When you hear the word scientist what comes to mind What do you think of when you hear
What do you think of when you hear You can argue
You can argue It can be seen in the front cover
It can be seen in the front cover