7 8 NOTES Exceptions to the Octet Rule


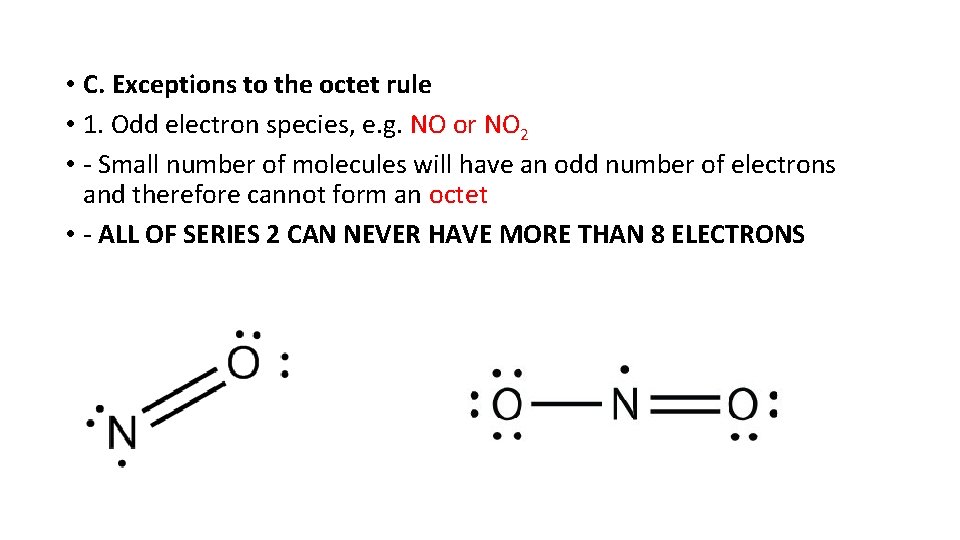





- Slides: 12

7. 8 NOTES Exceptions to the Octet Rule

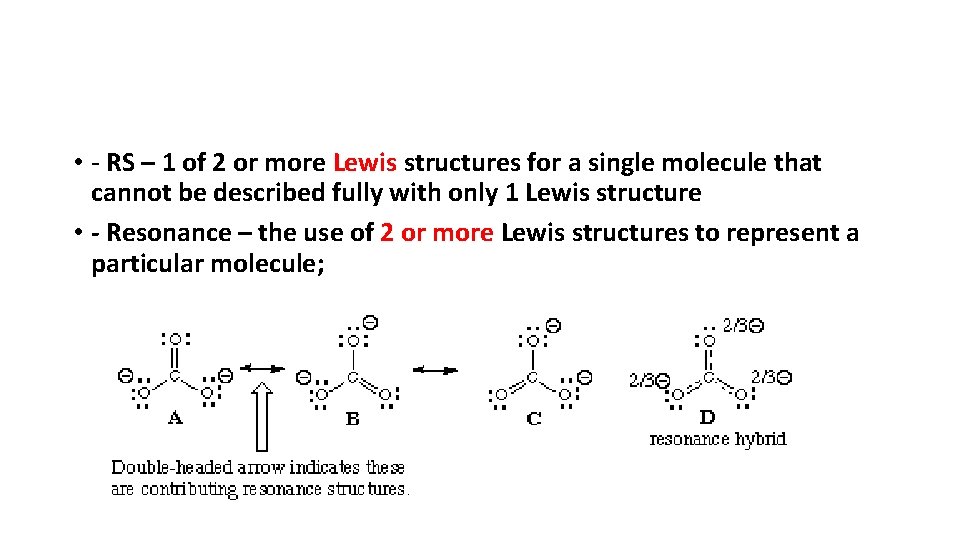
• B. Resonance structures –Certain molecules that are composed of single AND double bonds can be written in two different ways • - For example - O 3. • - If one is chosen over another it presents the problem of determining bond length; the single bond is longer than the double bond, but it has been proven both bonds in ozone have the same length • - Answer – use both structures to represent the molecule; known as resonance structures

• - RS – 1 of 2 or more Lewis structures for a single molecule that cannot be described fully with only 1 Lewis structure • - Resonance – the use of 2 or more Lewis structures to represent a particular molecule;

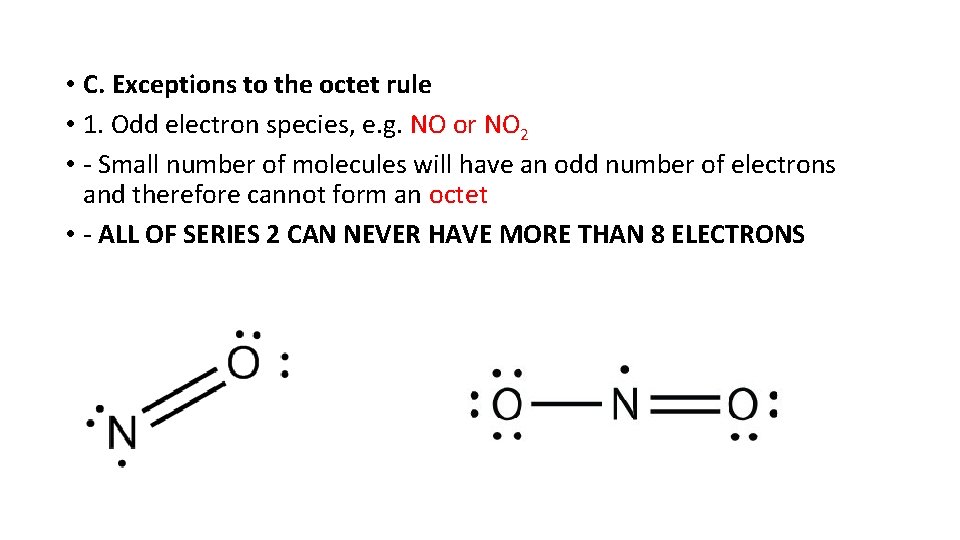
• C. Exceptions to the octet rule • 1. Odd electron species, e. g. NO or NO 2 • - Small number of molecules will have an odd number of electrons and therefore cannot form an octet • - ALL OF SERIES 2 CAN NEVER HAVE MORE THAN 8 ELECTRONS

2. Fewer than 8 electrons. BH 3 is the best example. Coordinate covalent bond: A covalent bond in which one of the atoms donates both electrons; some compounds have fewer than 8 electrons and tend to be reactive • BH 3 bonds with NH 3, with NH 3 donating both electrons creating a coordinate covalent bond • atoms or ions with lone pairs often form coordinate bonds; B, H, and halogens can never make a double bond b/c lack the # of orbitals • Examples: Be. H 2 H – Be – H; Be configuration 1 s 22 s 2; BX 3 where X is a halogen


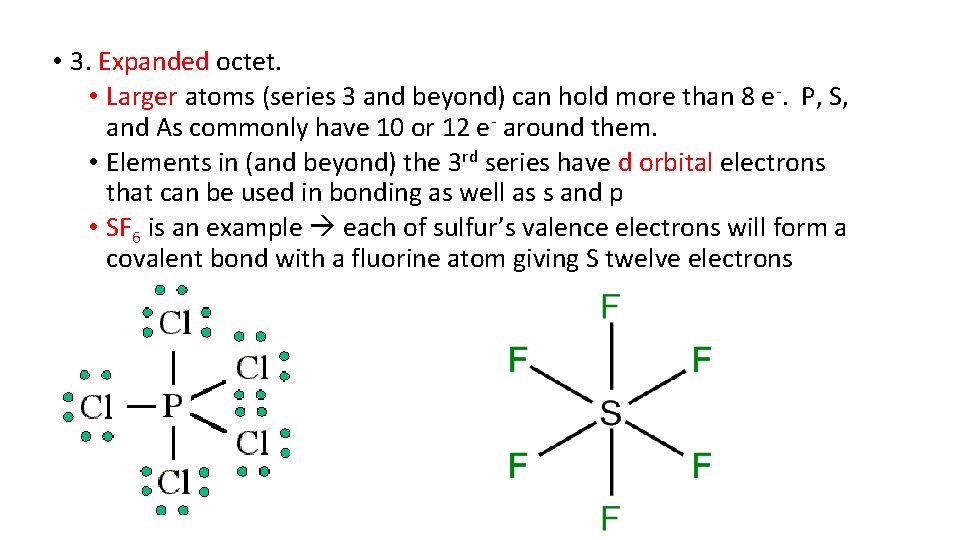
• 3. Expanded octet. • Larger atoms (series 3 and beyond) can hold more than 8 e-. P, S, and As commonly have 10 or 12 e- around them. • Elements in (and beyond) the 3 rd series have d orbital electrons that can be used in bonding as well as s and p • SF 6 is an example each of sulfur’s valence electrons will form a covalent bond with a fluorine atom giving S twelve electrons



