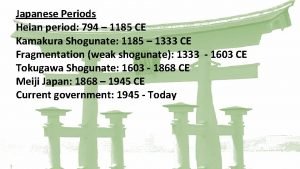
4152013 periods 1 3 5 4162013 periods 2







- Slides: 15

4/15/2013 (periods 1, 3, & 5) 4/16/2013 (periods 2 & 6) Standards: 5 (Acids and Bases) Objectives: ● Complete CST 83 -90 ● Students will be able to simplify ugly looking calculations DO NOW: 1. After turning in HW, Get responders and sign-in 2. Review how to calculate a log using my simple estimating log chart 3. CST Problems 83 -90 with responders (practice) HOMEWORK: NONE ============================== TURNED IN: ● Journal 4/12 (what you learned about how to calculate the log of a number with a power of 10) 1. Ch 19. 3 Ch 19. 2 p. 611 -613 19 (a, b, c); 20 (a, b, c) (5 pts) Std 4 2. CST Problems 77 -82 (5 pts) Std MISC (KEEP FOR NOTEBOOK)

For 4/16, see whiteboard for 4/15

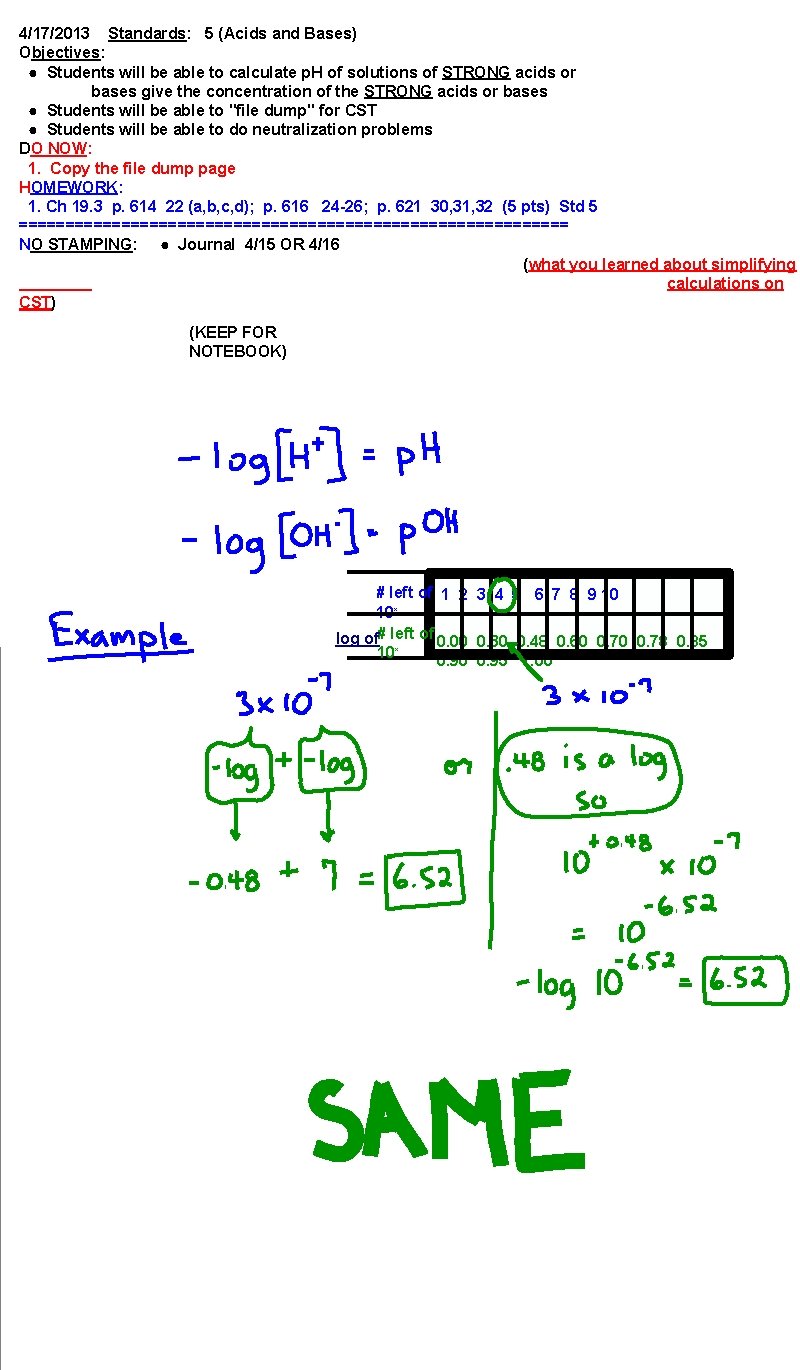
4/17/2013 Standards: 5 (Acids and Bases) Objectives: ● Students will be able to calculate p. H of solutions of STRONG acids or bases give the concentration of the STRONG acids or bases ● Students will be able to "file dump" for CST ● Students will be able to do neutralization problems DO NOW: 1. Copy the file dump page HOMEWORK: 1. Ch 19. 3 p. 614 22 (a, b, c, d); p. 616 24 -26; p. 621 30, 31, 32 (5 pts) Std 5 ============================== NO STAMPING: ● Journal 4/15 OR 4/16 (what you learned about simplifying calculations on CST) (KEEP FOR NOTEBOOK) # left of 1 2 3 4 5 6 7 8 9 10 10 x log of# left of 0. 00 0. 30 0. 48 0. 60 0. 78 0. 85 10 x 0. 90 0. 95 1. 00

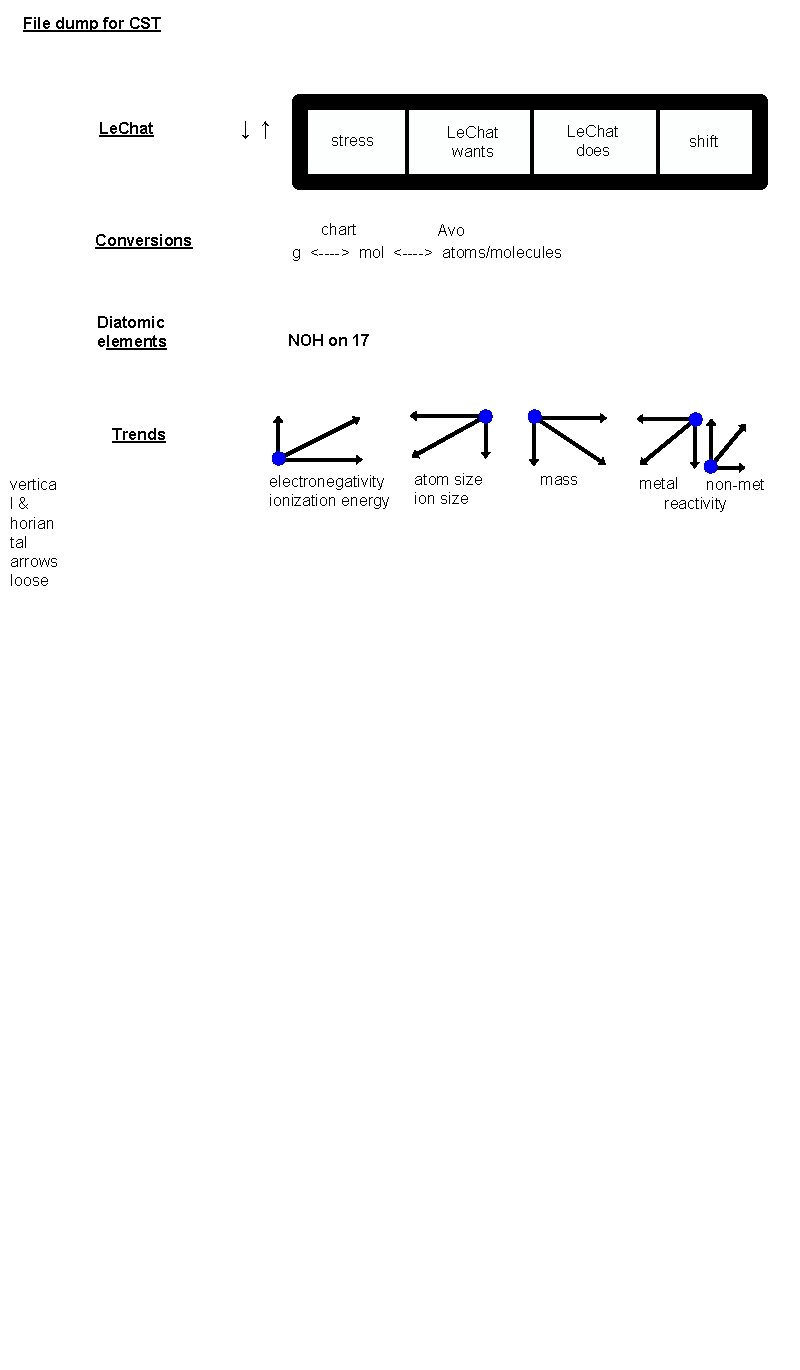
File dump for CST Le. Chat Conversions Diatomic elements ↓ ↑ stress Le. Chat does Le. Chat wants shift chart Avo g <----> mol <----> atoms/molecules NOH on 17 Trends vertica l & horian tal arrows loose electronegativity ionization energy atom size ion size mass metal non-met reactivity

Review of calculating p. H & p. OH

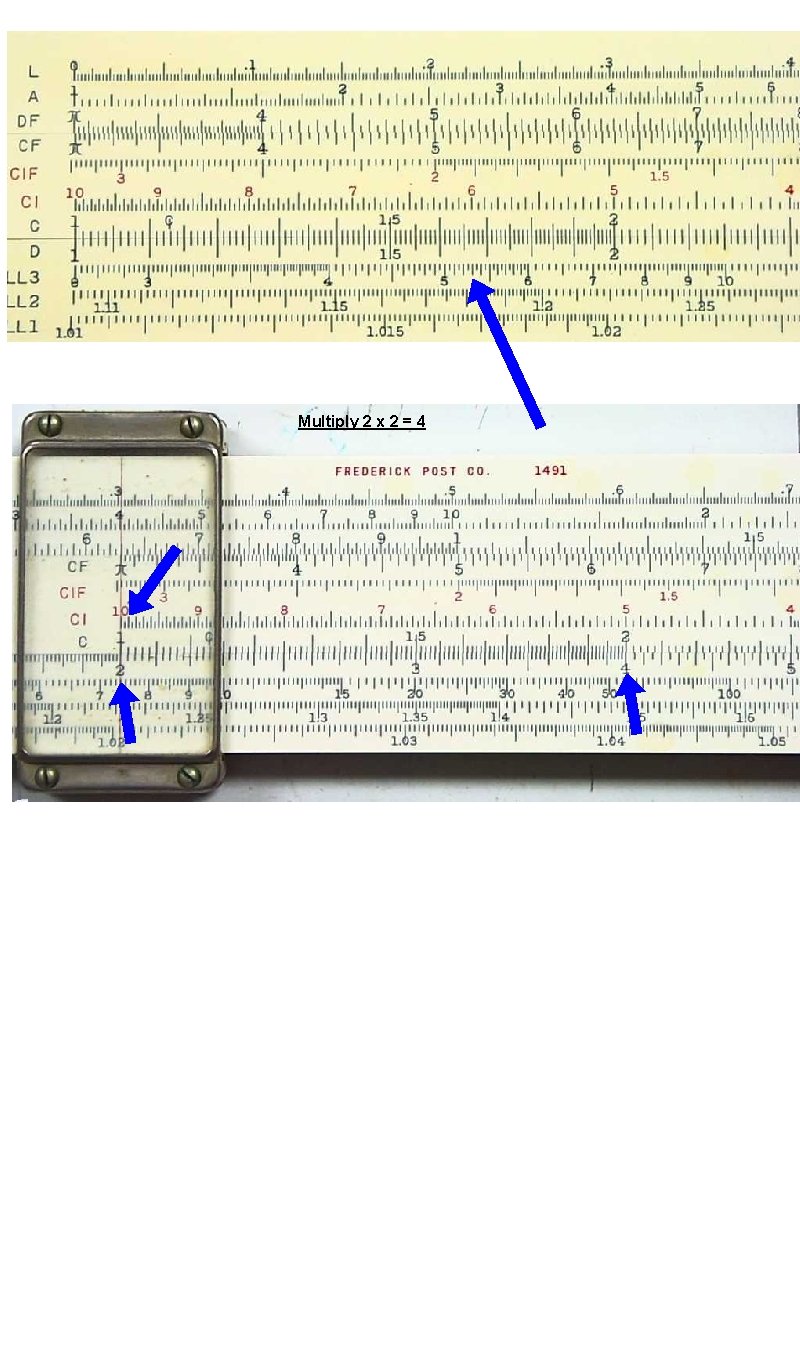
Multiply 2 x 2 = 4


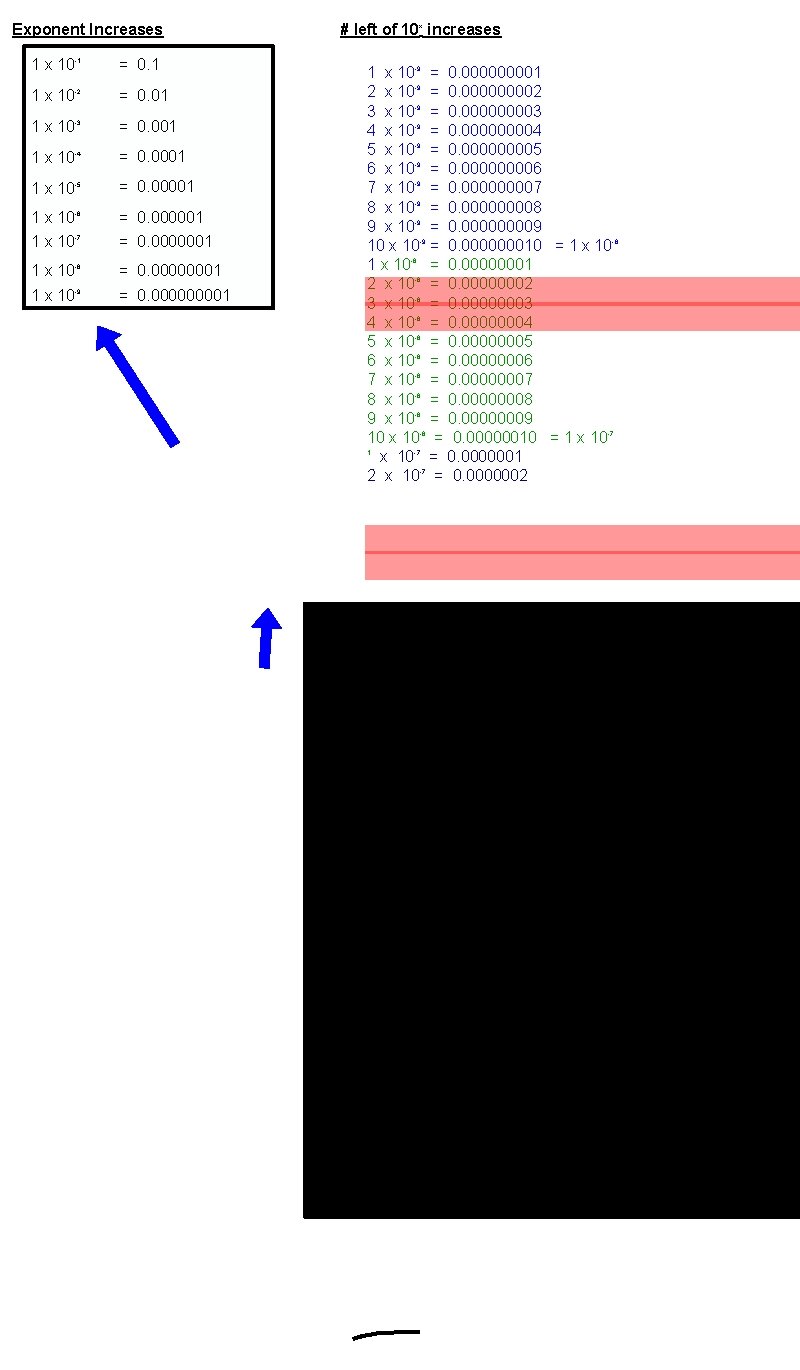
Exponent Increases 1 x 10 -1 = 0. 1 1 x 10 -2 = 0. 01 1 x 10 -3 = 0. 001 1 x 10 -4 = 0. 0001 1 x 10 -5 = 0. 00001 1 x 10 -6 1 x 10 -7 = 0. 000001 = 0. 0000001 1 x 10 -8 = 0. 00000001 1 x 10 -9 = 0. 00001 # left of 10 x increases 1 x 10 -9 = 0. 00001 2 x 10 -9 = 0. 00002 3 x 10 -9 = 0. 00003 4 x 10 -9 = 0. 00004 5 x 10 -9 = 0. 00005 6 x 10 -9 = 0. 00006 7 x 10 -9 = 0. 00007 8 x 10 -9 = 0. 00008 9 x 10 -9 = 0. 00009 10 x 10 -9 = 0. 000000010 = 1 x 10 -8 = 0. 00000001 2 x 10 -8 = 0. 00000002 3 x 10 -8 = 0. 00000003 4 x 10 -8 = 0. 00000004 5 x 10 -8 = 0. 00000005 6 x 10 -8 = 0. 00000006 7 x 10 -8 = 0. 00000007 8 x 10 -8 = 0. 00000008 9 x 10 -8 = 0. 00000009 10 x 10 -8 = 0. 00000010 = 1 x 10 -7 1 x 10 -7 = 0. 0000001 2 x 10 -7 = 0. 0000002

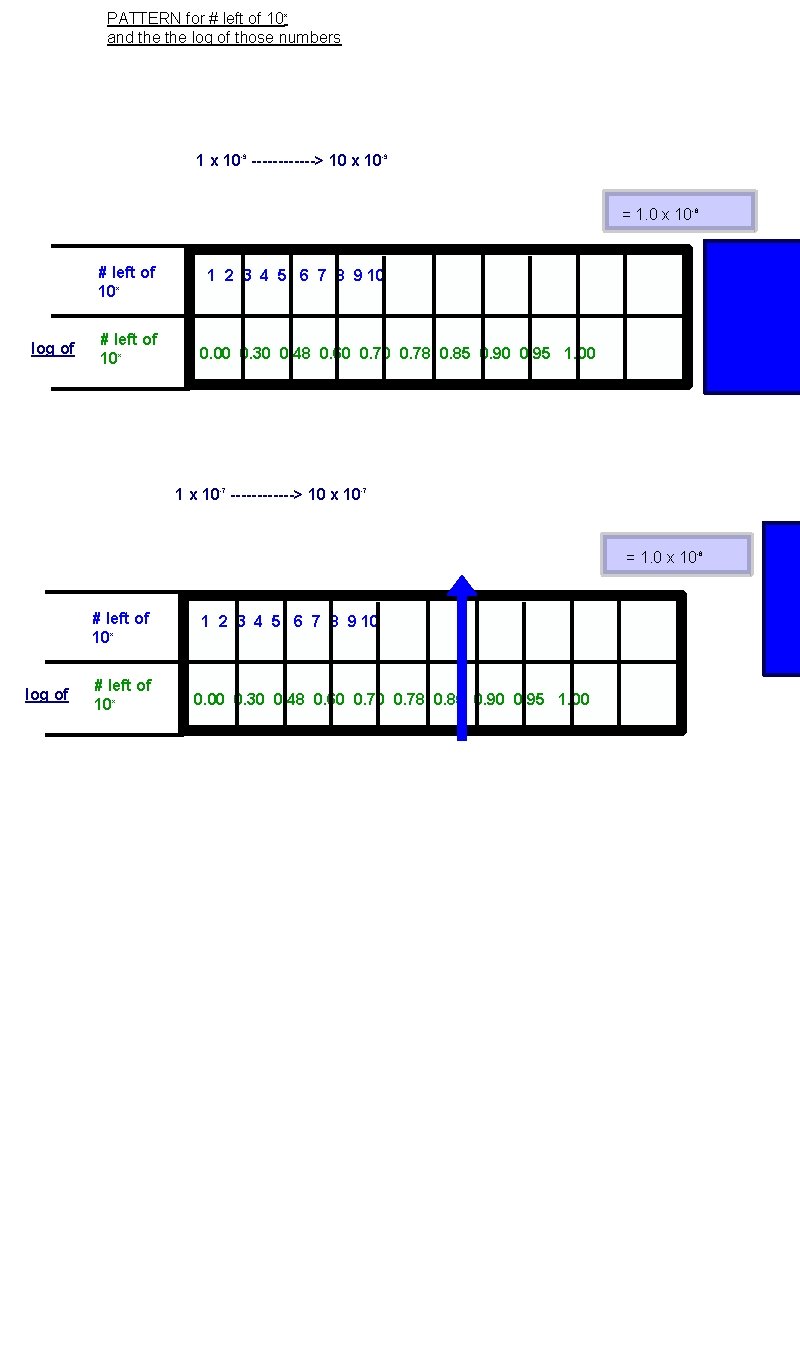
PATTERN for # left of 10 x and the log of those numbers 1 x 10 -9 ------> 10 x 10 -9 = 1. 0 x 10 -8 # left of 10 x log of # left of 10 x 1 2 3 4 5 6 7 8 9 10 0. 00 0. 30 0. 48 0. 60 0. 78 0. 85 0. 90 0. 95 1. 00 1 x 10 -7 ------> 10 x 10 -7 = 1. 0 x 10 -6 # left of 10 x log of # left of 10 x 1 2 3 4 5 6 7 8 9 10 0. 00 0. 30 0. 48 0. 60 0. 78 0. 85 0. 90 0. 95 1. 00

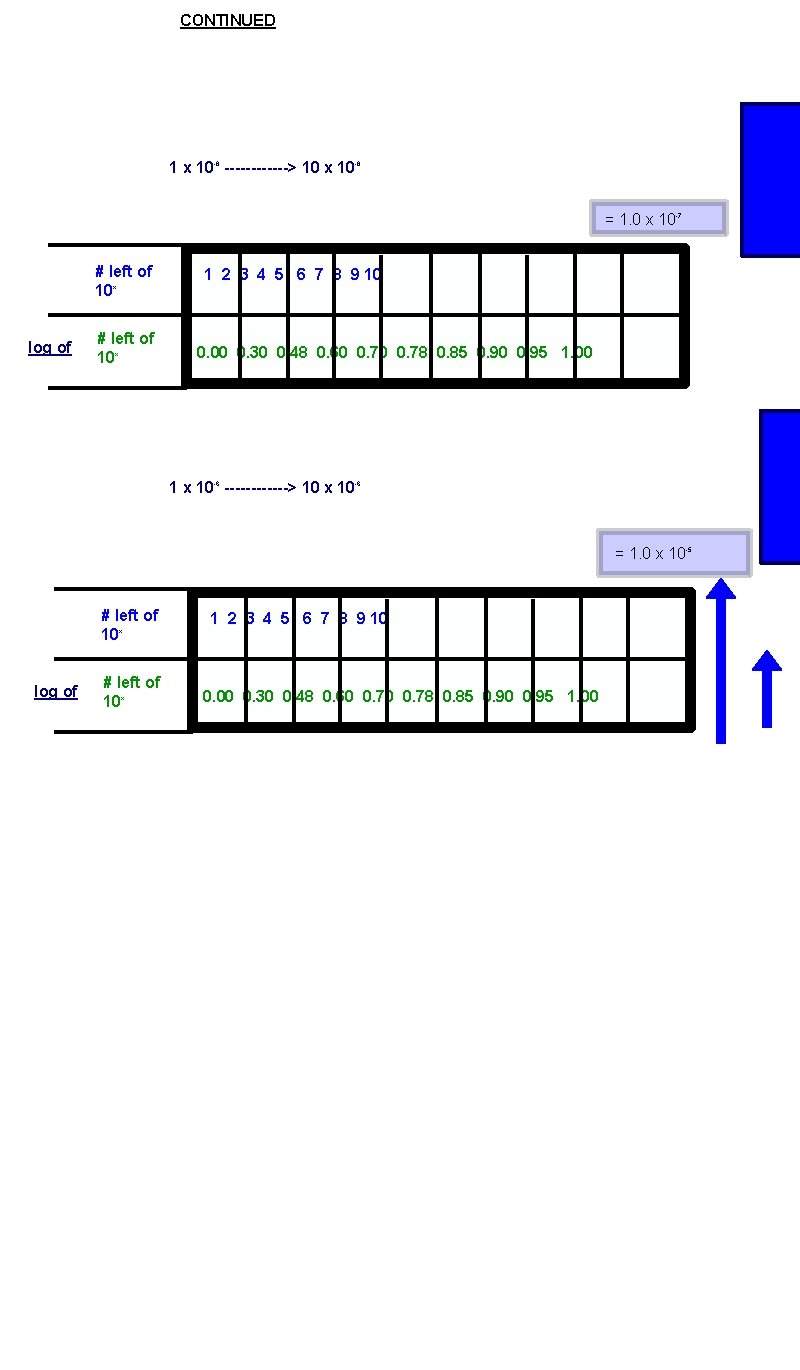
CONTINUED 1 x 10 -8 ------> 10 x 10 -8 = 1. 0 x 10 -7 # left of 10 x log of # left of 10 x 1 2 3 4 5 6 7 8 9 10 0. 00 0. 30 0. 48 0. 60 0. 78 0. 85 0. 90 0. 95 1. 00 1 x 10 -6 ------> 10 x 10 -6 = 1. 0 x 10 -5 # left of 10 x log of # left of 10 x 1 2 3 4 5 6 7 8 9 10 0. 00 0. 30 0. 48 0. 60 0. 78 0. 85 0. 90 0. 95 1. 00

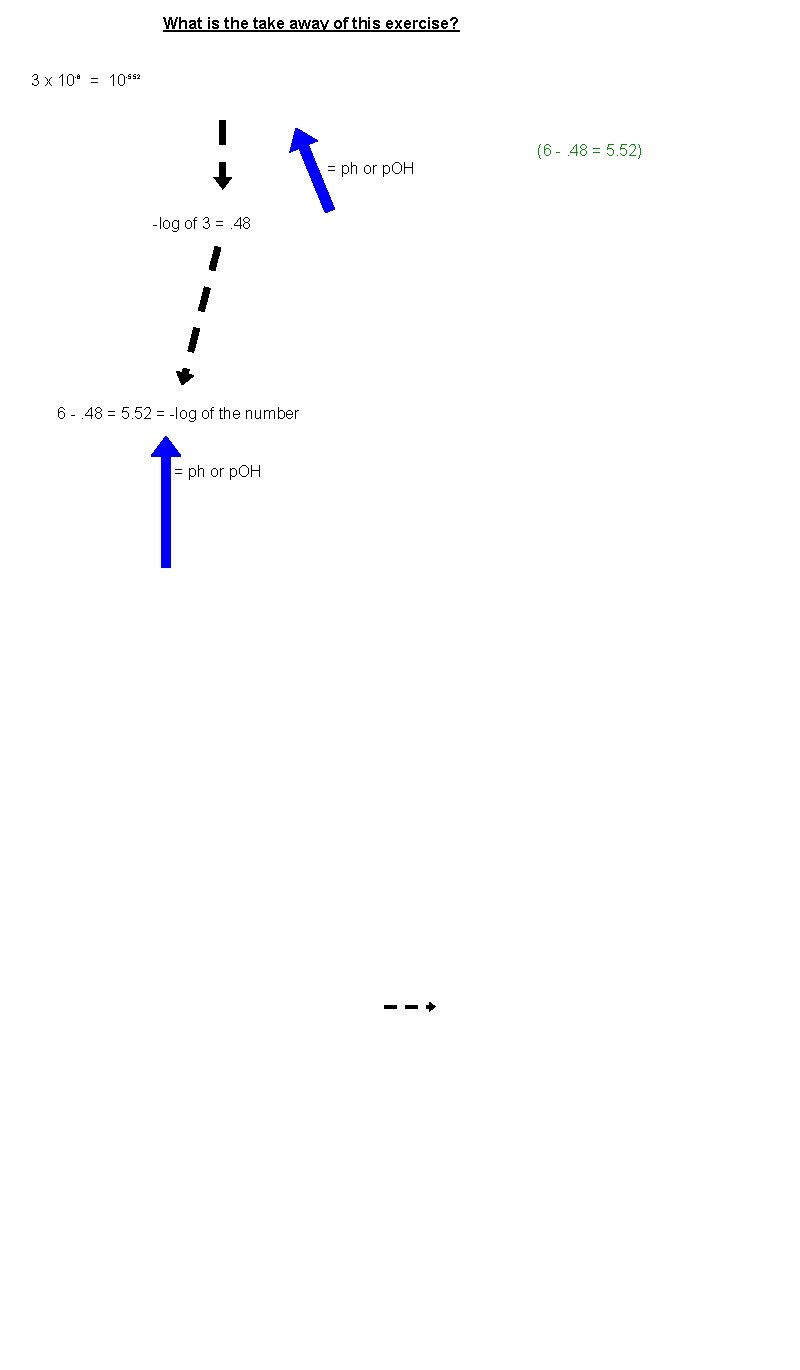
What is the take away of this exercise? 3 x 10 -6 = 10 -5. 52 (6 -. 48 = 5. 52) = ph or p. OH -log of 3 =. 48 6 -. 48 = 5. 52 = -log of the number = ph or p. OH

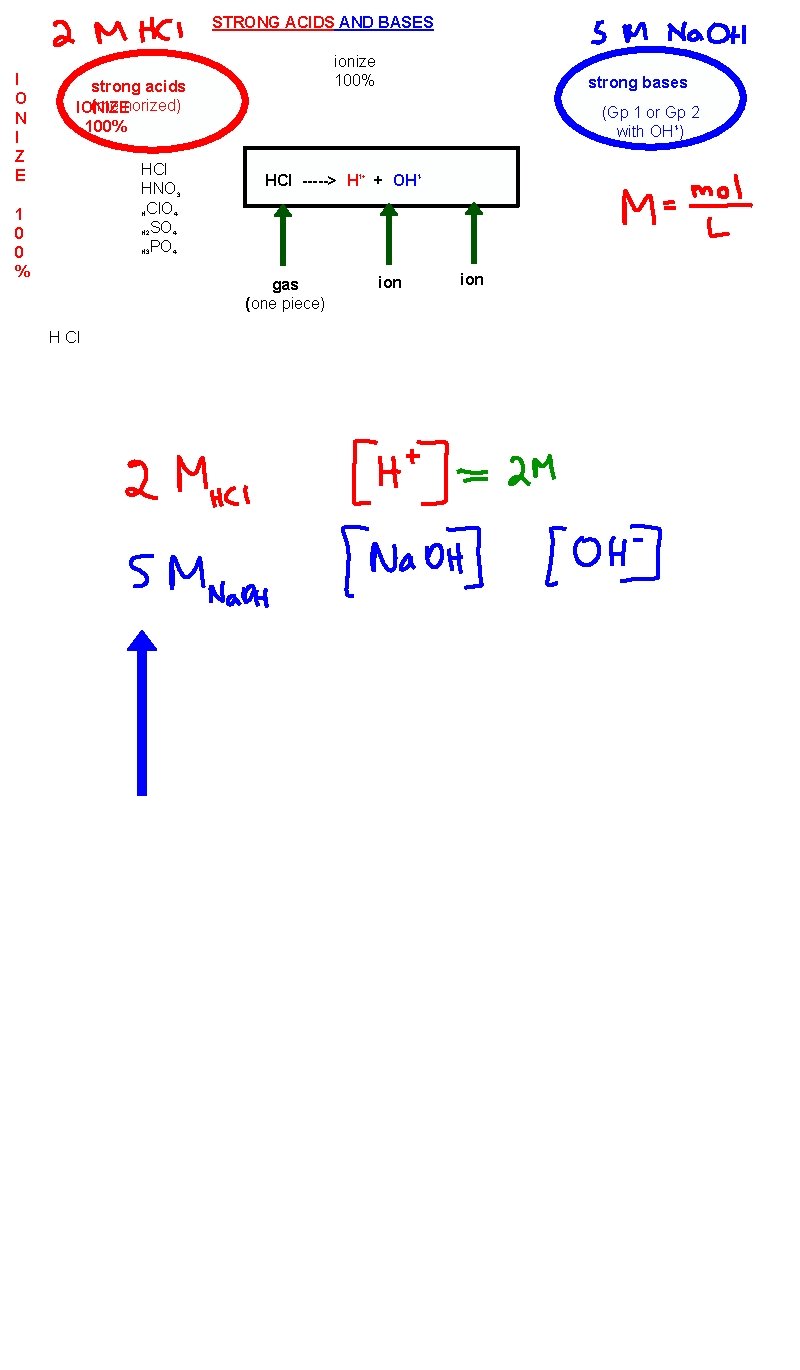
STRONG ACIDS AND BASES I O N I Z E 1 0 0 % ionize 100% strong acids (memorized) IONIZE 100% HCl HNO 3 HCl. O 4 H 2 SO 4 H 3 PO 4 (Gp 1 or Gp 2 with OH 1 -) HCl -----> H 1+ + OH 1 - gas (one piece) H Cl strong bases ion

M 1 V 1 a = M 2 V 2 b

4/18/2013 Standards: Biology - NCLB practice Objectives: ● Students will perform well for the State NCLB Bio Test DO NOW: 1. Read the NCLB background answer as many of the questions as you can. Practice Quiz due end of period, you keep background notes. HOMEWORK: 1. ============================== TURN IN: ● Journal 4/17 (Calculating p. H & p. OH) (KEEP FOR NOTEBOOK)

 Curritage
Curritage British literature timeline
British literature timeline A cricket match is divided into periods called
A cricket match is divided into periods called The approximate dates of the baroque period are
The approximate dates of the baroque period are Dua for dead person
Dua for dead person Topic 15 periods authors and genres
Topic 15 periods authors and genres Literary time periods
Literary time periods Old english period
Old english period 1965 literary timeline
1965 literary timeline Normal head circumference of newborn
Normal head circumference of newborn Dinosaur time periods
Dinosaur time periods English literary periods and their characteristics
English literary periods and their characteristics Western music periods
Western music periods Realism definition
Realism definition Hair alternates between periods of growth and rest
Hair alternates between periods of growth and rest Four periods of history
Four periods of history