10 2 NOTES Pressure Pressure force over area



- Slides: 10

10. 2 NOTES Pressure

• Pressure- force over area that force is applied on • • Less area with same force = greater pressure • • Measured in pascals (Pa), which is the SI unit for pressure • • These are very small, so kilopascals, or k. Pa, are used, which are 1000 times greater in size than Pa

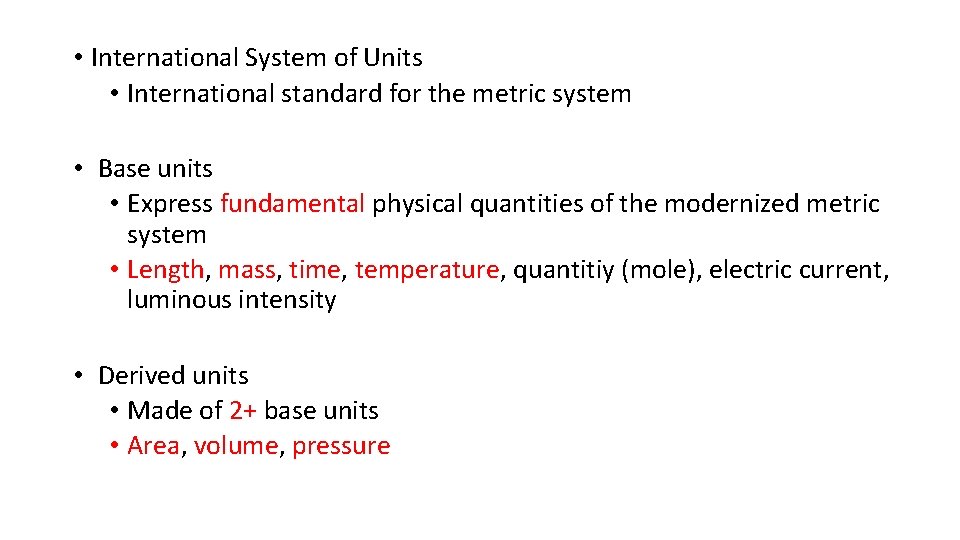
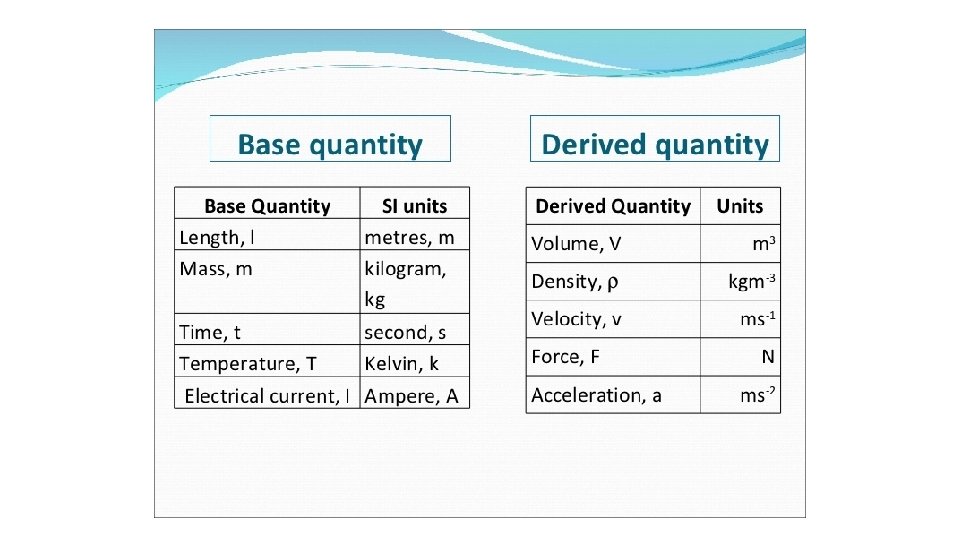
• International System of Units • International standard for the metric system • Base units • Express fundamental physical quantities of the modernized metric system • Length, mass, time, temperature, quantitiy (mole), electric current, luminous intensity • Derived units • Made of 2+ base units • Area, volume, pressure


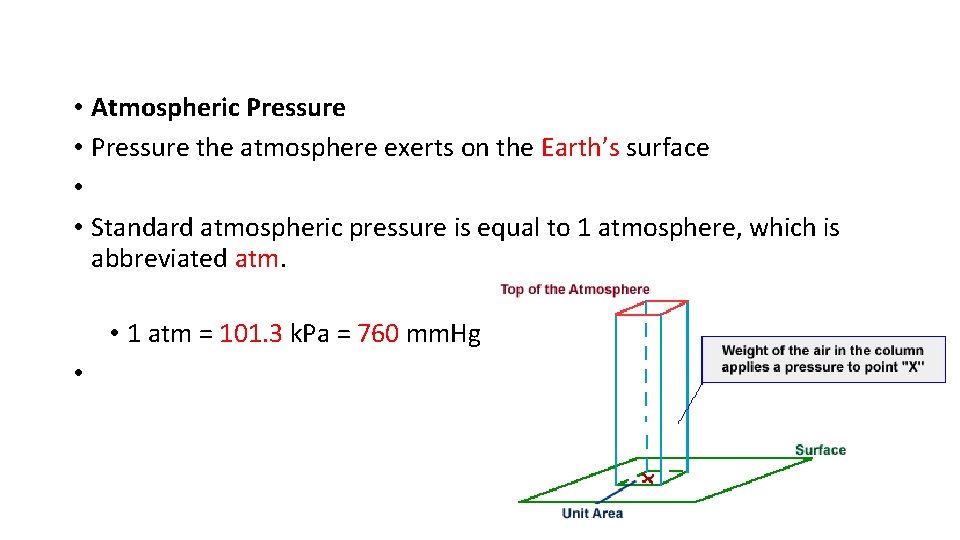
• Atmospheric Pressure • Pressure the atmosphere exerts on the Earth’s surface • • Standard atmospheric pressure is equal to 1 atmosphere, which is abbreviated atm. • 1 atm = 101. 3 k. Pa = 760 mm. Hg •

• Barometer- device for measuring atmospheric pressure •

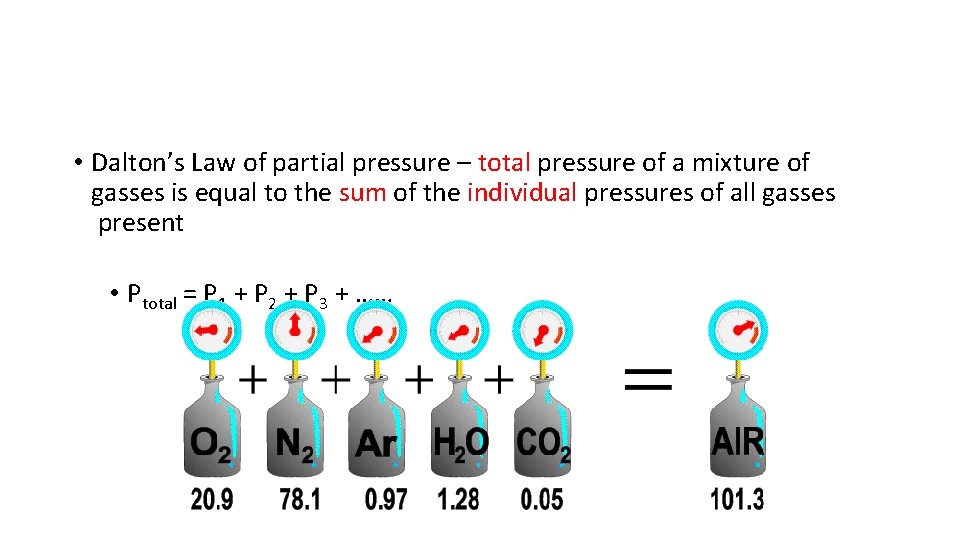
• Dalton’s Law of partial pressure – total pressure of a mixture of gasses is equal to the sum of the individual pressures of all gasses present • Ptotal = P 1 + P 2 + P 3 + ……

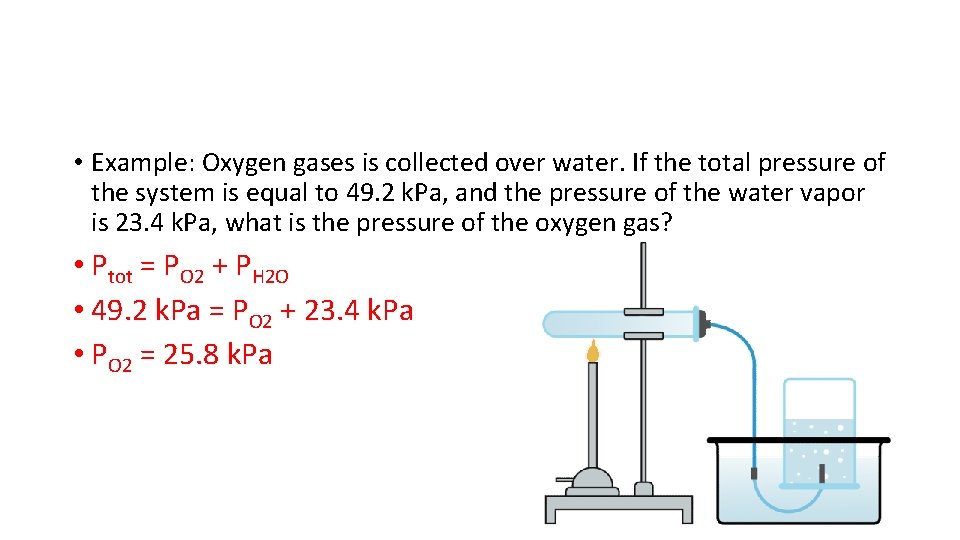
• Example: Oxygen gases is collected over water. If the total pressure of the system is equal to 49. 2 k. Pa, and the pressure of the water vapor is 23. 4 k. Pa, what is the pressure of the oxygen gas? • Ptot = PO 2 + PH 2 O • 49. 2 k. Pa = PO 2 + 23. 4 k. Pa • PO 2 = 25. 8 k. Pa

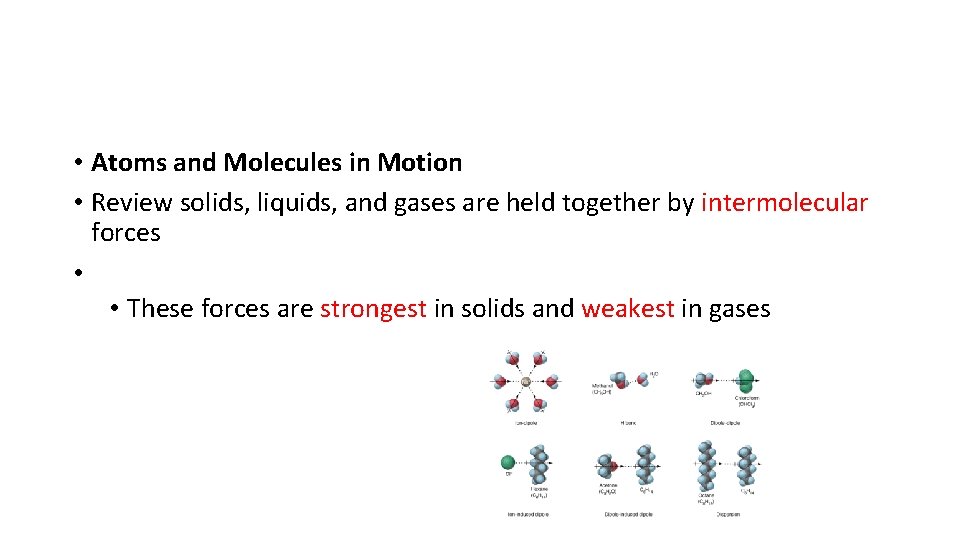
• Atoms and Molecules in Motion • Review solids, liquids, and gases are held together by intermolecular forces • • These forces are strongest in solids and weakest in gases

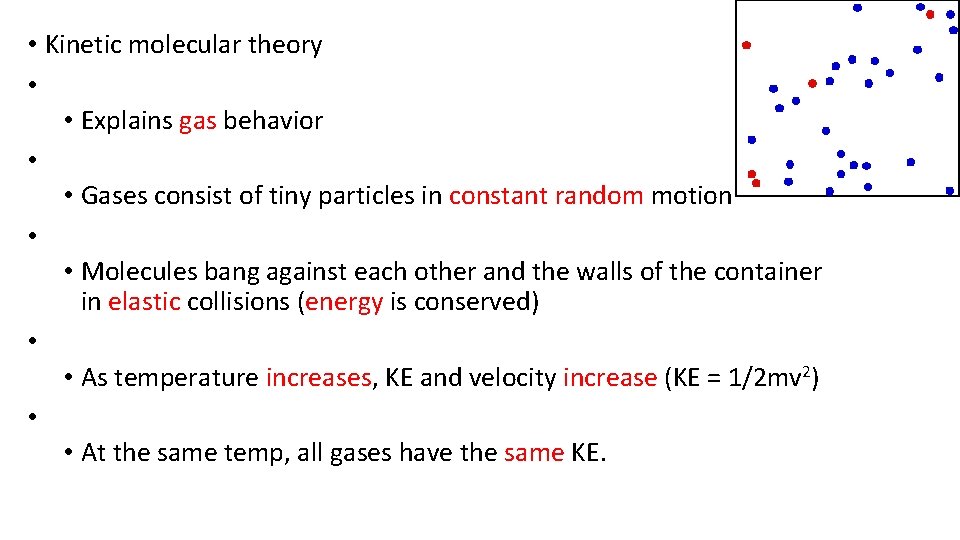
• Kinetic molecular theory • • Explains gas behavior • • Gases consist of tiny particles in constant random motion • • Molecules bang against each other and the walls of the container in elastic collisions (energy is conserved) • • As temperature increases, KE and velocity increase (KE = 1/2 mv 2) • • At the same temp, all gases have the same KE.