1 Solutions Why does a raw egg swell










- Slides: 17

1

Solutions Why does a raw egg swell or shrink when placed in different solutions? 2

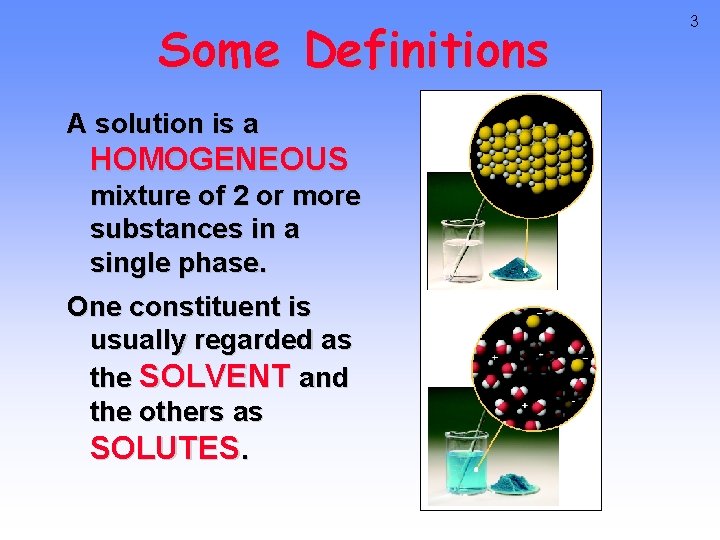
Some Definitions A solution is a HOMOGENEOUS mixture of 2 or more substances in a single phase. One constituent is usually regarded as the SOLVENT and the others as SOLUTES. 3

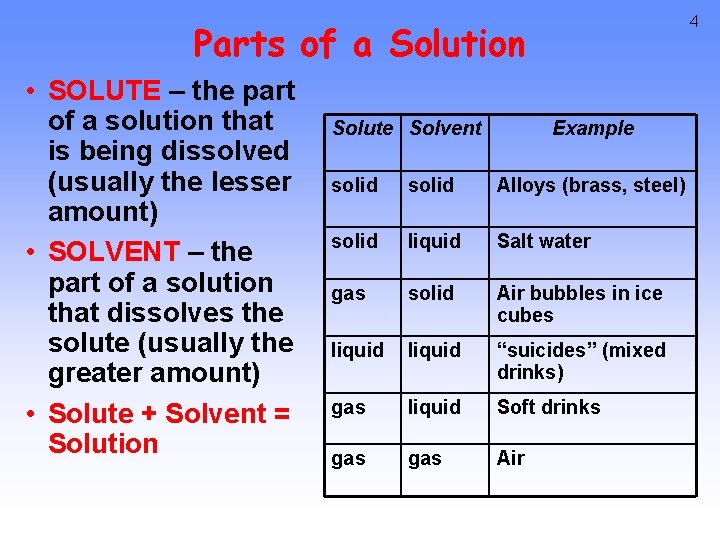
4 Parts of a Solution • SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) • SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) • Solute + Solvent = Solution Solute Solvent Example solid Alloys (brass, steel) solid liquid Salt water gas solid Air bubbles in ice cubes liquid “suicides” (mixed drinks) gas liquid Soft drinks gas Air

IONIC COMPOUNDS Dissolve in water! Create Aqueous Solutions KMn. O 4 in water K+(aq) + Mn. O 4 -(aq) 5

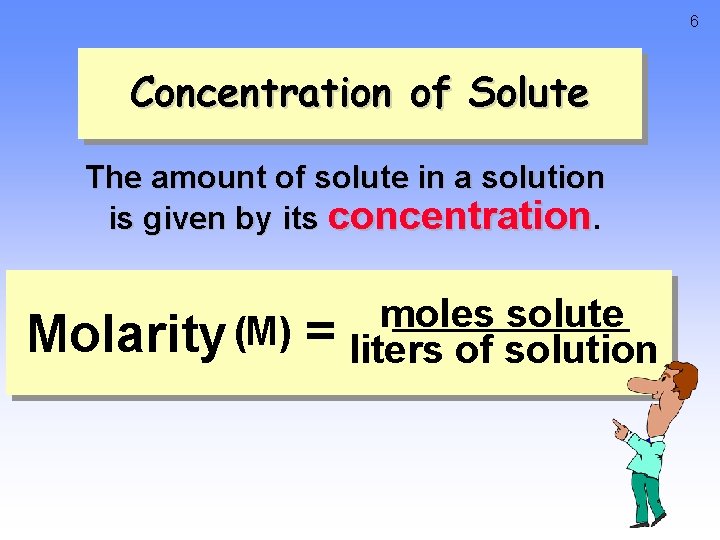
6 Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution

7 Think of O. J! • Do you like Concentrated Orange Juice? • What does that mean? • What if we could describe the amount of Orange Juice in a solution. • That’s why we have Molarity!!!

To make a 1 M solution of Copper (II) Sulfate Add 1 Mole of Copper Sulfate Add Enough water so that the SOLUTION has a volume of 1 L 8

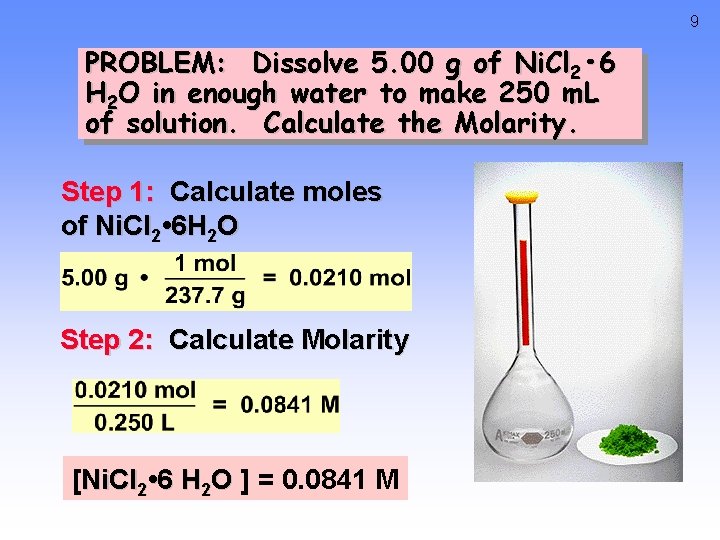
9 PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate the Molarity. Step 1: Calculate moles of Ni. Cl 2 • 6 H 2 O Step 2: Calculate Molarity [Ni. Cl 2 • 6 H 2 O ] = 0. 0841 M

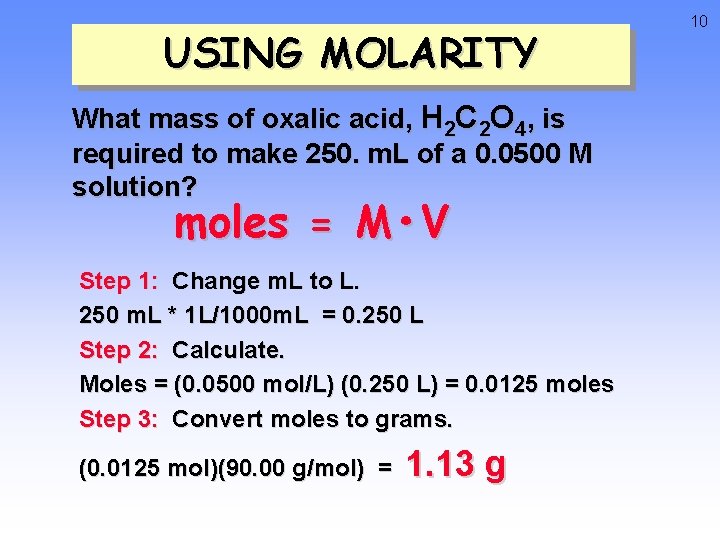
USING MOLARITY What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? moles = M • V Step 1: Change m. L to L. 250 m. L * 1 L/1000 m. L = 0. 250 L Step 2: Calculate. Moles = (0. 0500 mol/L) (0. 250 L) = 0. 0125 moles Step 3: Convert moles to grams. (0. 0125 mol)(90. 00 g/mol) = 1. 13 g 10

11 Learning Check How many grams of Na. OH are required to prepare 400. m. L of 3. 0 M Na. OH solution? 1) 12 g 2) 48 g 3) 300 g

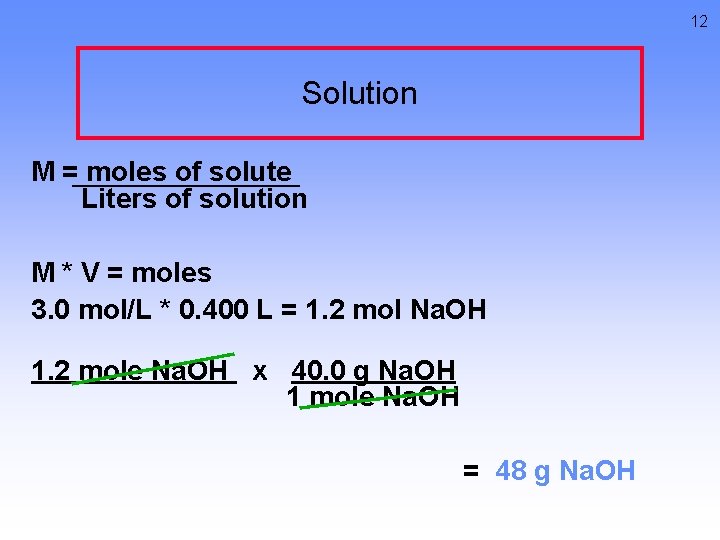
12 Solution M = moles of solute Liters of solution M * V = moles 3. 0 mol/L * 0. 400 L = 1. 2 mol Na. OH 1. 2 mole Na. OH x 40. 0 g Na. OH 1 mole Na. OH = 48 g Na. OH

13 Preparing Solutions • Weigh out a solid solute and dissolve in a given quantity of solvent.

14 Mixing Solutions How would you make 500. m. L of a 2. 50 M Na. OH solution? Step 1: Convert volume m. L to L. 500. m. L * 1 L/1000 m. L =. 500 L Step 2: Calculate number of moles Na. OH needed. moles = M * liters moles = 2. 50 M *. 500 L = 1. 25 moles Na. OH

15 How can you “Dilute Solutions” • MV=MV How would you make 100 m. L of a. 1 M Na. OH solution from a 1 M stock solution? (. 1 L)(. 1 M/L) =1 M X 1 M. 01 L or 10 m. L and add 90 m. L of water

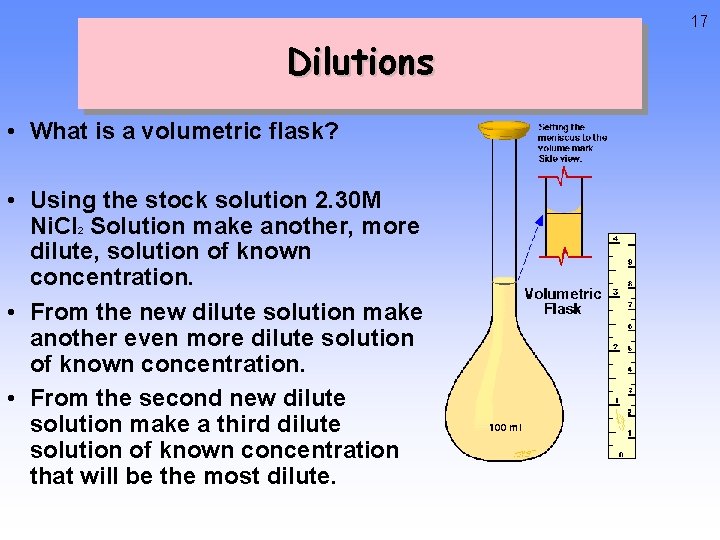
16 Your tasks… • Make 20 m. L of a. 100 M Sucrose solution • Create 30 m. L of a. 2 M solution from a Stock solution of 2 Molar Iodine Solution

17 Dilutions • What is a volumetric flask? • Using the stock solution 2. 30 M Ni. Cl 2 Solution make another, more dilute, solution of known concentration. • From the new dilute solution make another even more dilute solution of known concentration. • From the second new dilute solution make a third dilute solution of known concentration that will be the most dilute.