Supplemental Power Point Slides Spinal Sealant System Provides


- Slides: 5

Supplemental Power. Point Slides Spinal Sealant System Provides Better Intraoperative Watertight Closure than Standard of Care during Spinal Surgery: A Prospective, Multi-Center, Randomized Controlled Study Neill M. Wright, MD 1; Jon Park, MD 2; John M. Tew, MD 3; Kee D. Kim, MD 4; Mark E. Shaffrey, MD 5; Joseph Cheng, MD 6; Haroon Choudhri, MD 7; Ajit A. Krishnaney, MD 8; R. Scott Graham, MD 9; Ehud Mendel, MD 10; Nathan Simmons, MD 11 From: 1. Washington University School of Medicine, St. Louis, MO; 2. Stanford University Medical Center, Stanford, CA; 3. University of Cincinnati/The Mayfield Clinic, Cincinnati, OH; 4. University of California Davis, Sacramento, CA; 5. University of Virginia Health Systems, Charlottesville, VA; 6. Vanderbilt University Medical Center, Nashville, TN; 7. Medical College of Georgia, Augusta, GA; 8. Cleveland Clinic, Cleveland, OH; 9. Virginia Commonwealth University, Richmond, VA; 10. Ohio State University Medical Center; Columbus, OH; 11. Dartmouth-Hitchcock Medical Center, Lebanon, NH Copyright © 2015 Lippincott Williams & Wilkins. Unauthorized commercial reproduction of this slide is prohibited

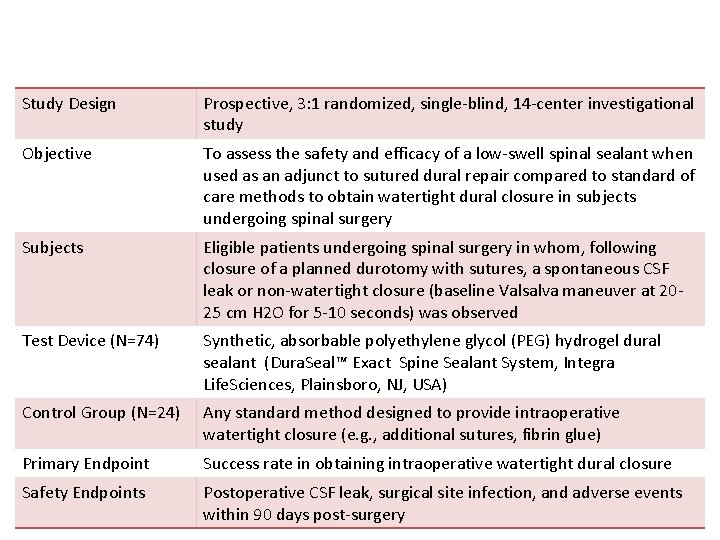
Study Design Prospective, 3: 1 randomized, single-blind, 14 -center investigational study Objective To assess the safety and efficacy of a low-swell spinal sealant when used as an adjunct to sutured dural repair compared to standard of care methods to obtain watertight dural closure in subjects undergoing spinal surgery Subjects Eligible patients undergoing spinal surgery in whom, following closure of a planned durotomy with sutures, a spontaneous CSF leak or non-watertight closure (baseline Valsalva maneuver at 2025 cm H 2 O for 5 -10 seconds) was observed Test Device (N=74) Synthetic, absorbable polyethylene glycol (PEG) hydrogel dural sealant (Dura. Seal™ Exact Spine Sealant System, Integra Life. Sciences, Plainsboro, NJ, USA) Control Group (N=24) Any standard method designed to provide intraoperative watertight closure (e. g. , additional sutures, fibrin glue) Primary Endpoint Success rate in obtaining intraoperative watertight dural closure Safety Endpoints Postoperative CSF leak, surgical site infection, and adverse events within 90 days post-surgery

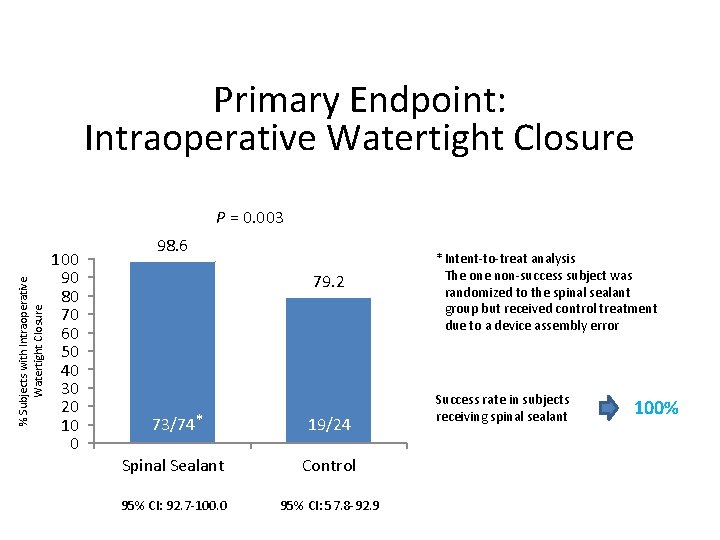
Primary Endpoint: Intraoperative Watertight Closure % Subjects with Intraoperative Watertight Closure P = 0. 003 100 90 80 70 60 50 40 30 20 10 0 98. 6 79. 2 73/74* 19/24 Spinal Sealant Control 95% CI: 92. 7 -100. 0 95% CI: 57. 8 -92. 9 * Intent-to-treat analysis The one non-success subject was randomized to the spinal sealant group but received control treatment due to a device assembly error Success rate in subjects receiving spinal sealant 100%

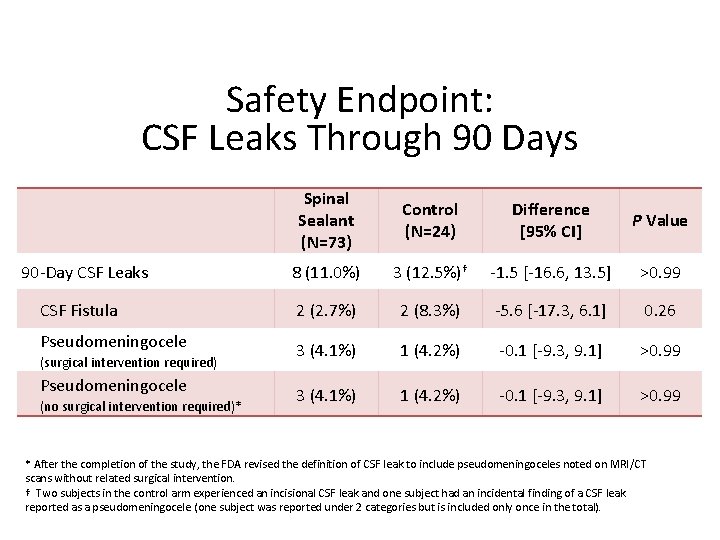
Safety Endpoint: CSF Leaks Through 90 Days 90 -Day CSF Leaks CSF Fistula Pseudomeningocele (surgical intervention required) Pseudomeningocele (no surgical intervention required)* Spinal Sealant (N=73) Control (N=24) Difference [95% CI] P Value 8 (11. 0%) 3 (12. 5%)† -1. 5 [-16. 6, 13. 5] >0. 99 2 (2. 7%) 2 (8. 3%) -5. 6 [-17. 3, 6. 1] 0. 26 3 (4. 1%) 1 (4. 2%) -0. 1 [-9. 3, 9. 1] >0. 99 * After the completion of the study, the FDA revised the definition of CSF leak to include pseudomeningoceles noted on MRI/CT scans without related surgical intervention. † Two subjects in the control arm experienced an incisional CSF leak and one subject had an incidental finding of a CSF leak reported as a pseudomeningocele (one subject was reported under 2 categories but is included only once in the total).

Conclusions • This study demonstrates the efficacy and safety of a low-swell formulation of a hydrogel PEG sealant as an adjunct to sutures in achieving watertight dural closure in durotomies. • An on-going post-market study is assessing the safety and efficacy of this hydrogel sealant in both intentional and incidental durotomies.