1 mole Molar Volume the volume of STP
- Slides: 5

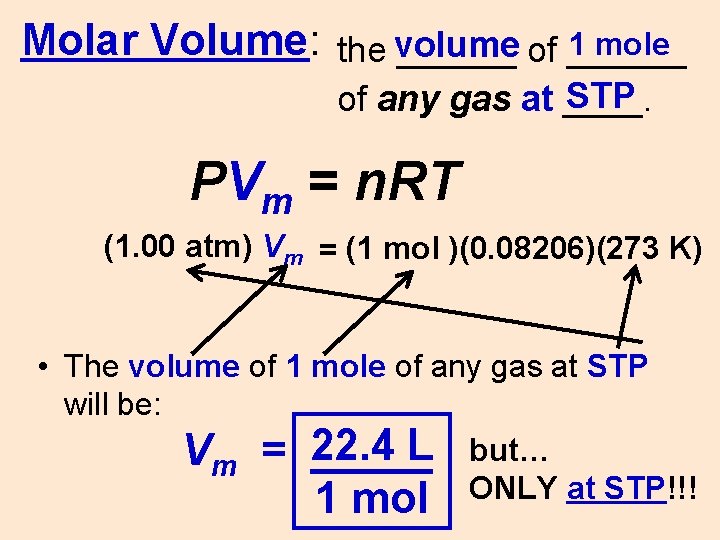
1 mole Molar Volume: the volume ______ of ______ STP of any gas at ____. PVm = n. RT (1. 00 atm) Vm = (1 mol )(0. 08206)(273 K) • The volume of 1 mole of any gas at STP will be: L but… Vm = 22. 4 _____ ONLY at STP!!! 1 mol

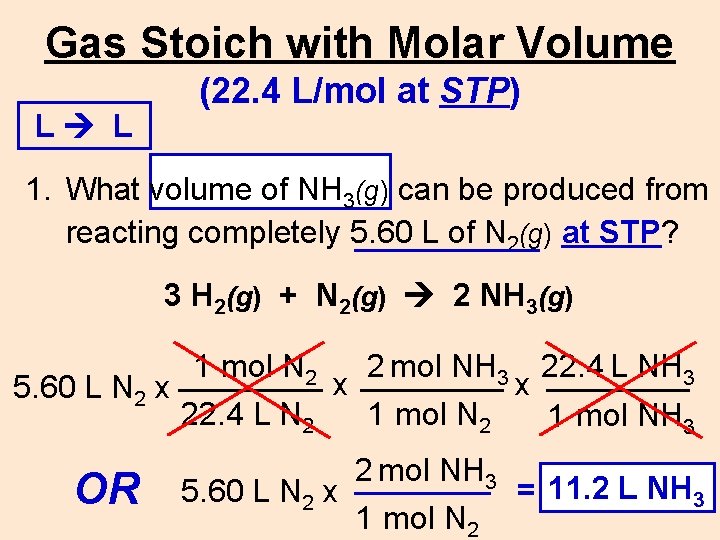
Gas Stoich with Molar Volume L L (22. 4 L/mol at STP) 1. What volume of NH 3(g) can be produced from reacting completely 5. 60 L of N 2(g) at STP? 3 H 2(g) + N 2(g) 2 NH 3(g) 1 mol N 2 2 mol NH 3 22. 4 L NH 3 x x 5. 60 L N 2 x 22. 4 L N 2 1 mol NH 3 OR 5. 60 L N 2 x 2 mol NH 3 1 mol N 2 11. 2 L NH 33 = ____L

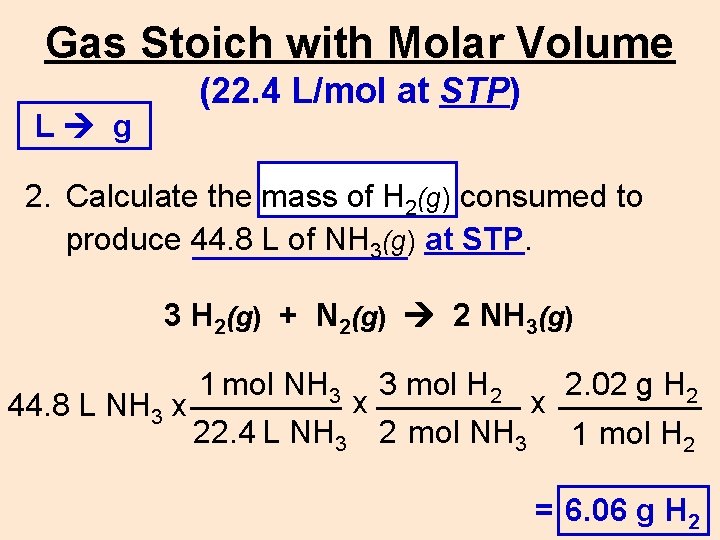
Gas Stoich with Molar Volume L g (22. 4 L/mol at STP) 2. Calculate the mass of H 2(g) consumed to produce 44. 8 L of NH 3(g) at STP. 3 H 2(g) + N 2(g) 2 NH 3(g) 1 mol NH 3 3 mol H 2 2. 02 g H 2 x x 44. 8 L NH 3 x 22. 4 L NH 3 2 mol NH 3 1 mol H 2 = ____g 6. 06 g H 22

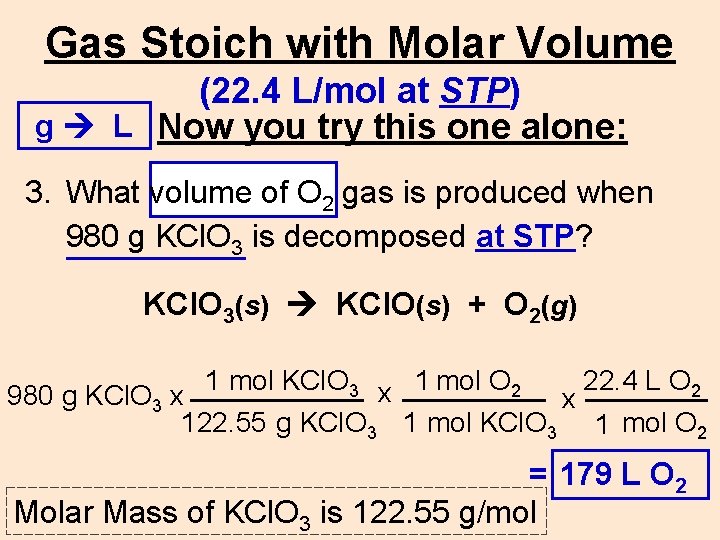
Gas Stoich with Molar Volume (22. 4 L/mol at STP) g L Now you try this one alone: 3. What volume of O 2 gas is produced when 980 g KCl. O 3 is decomposed at STP? KCl. O 3(s) KCl. O(s) + O 2(g) 1 mol KCl. O 3 x 1 mol O 2 22. 4 L O 2 980 g KCl. O 3 x x 122. 55 g KCl. O 3 1 mol O 2 = ____L 179 L O O 22 Molar Mass of KCl. O 3 is 122. 55 g/mol

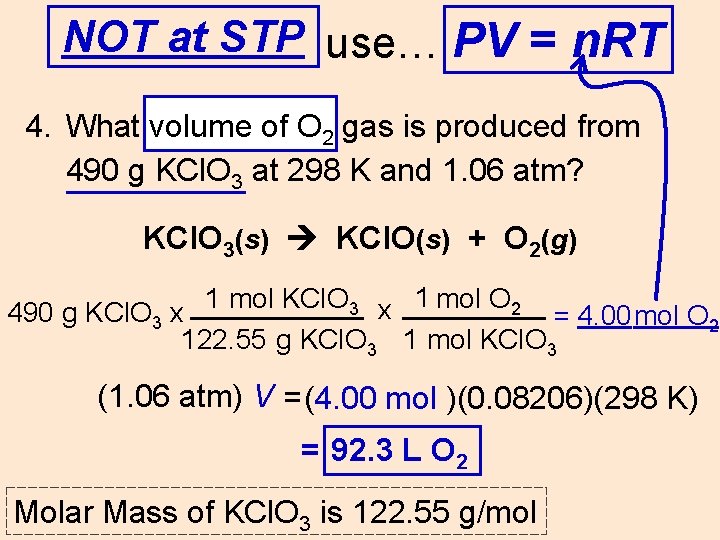
NOT at STP use… PV = n. RT 4. What volume of O 2 gas is produced from 490 g KCl. O 3 at 298 K and 1. 06 atm? KCl. O 3(s) KCl. O(s) + O 2(g) 1 mol KCl. O 3 x 1 mol O 2 490 g KCl. O 3 x = 4. 00 mol O 2 122. 55 g KCl. O 3 1 mol KCl. O 3 (1. 06 atm) V =(4. 00 mol )(0. 08206)(298 K) = ____L 92. 3 L O 22 Molar Mass of KCl. O 3 is 122. 55 g/mol