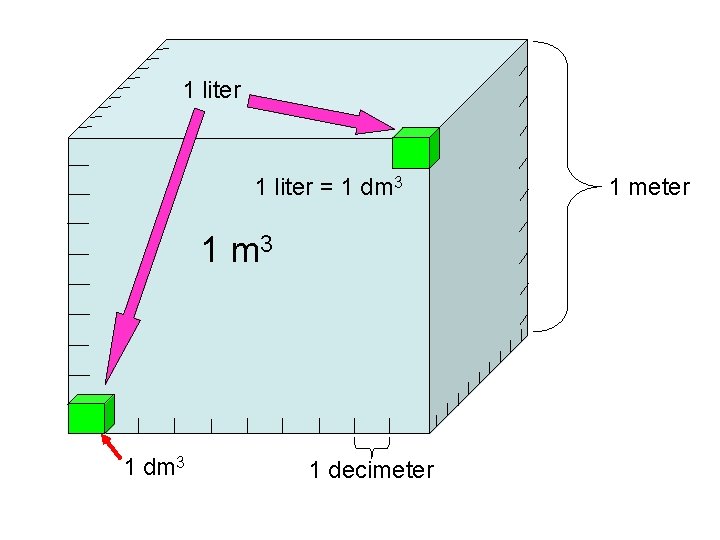
1 liter 1 dm 3 1 decimeter 1


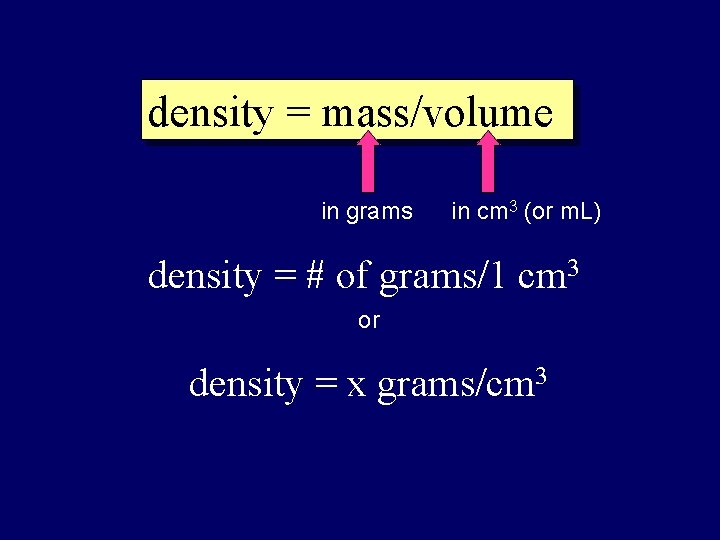
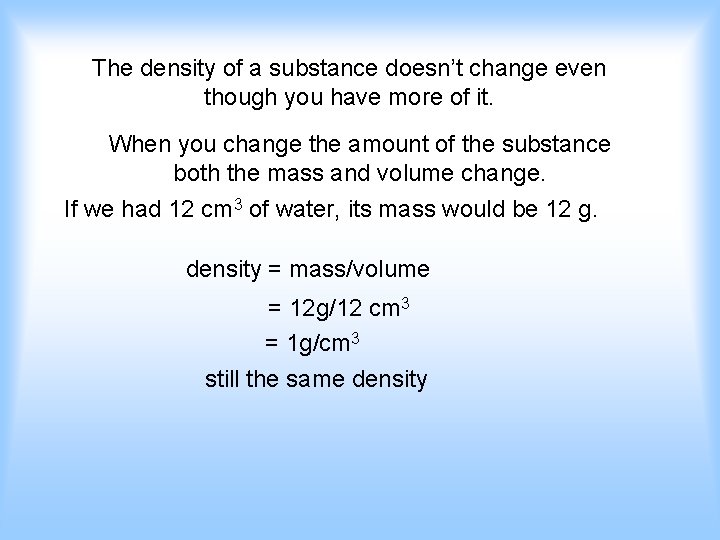

- Slides: 20


1 liter = 1 dm 3 1 decimeter 1 meter

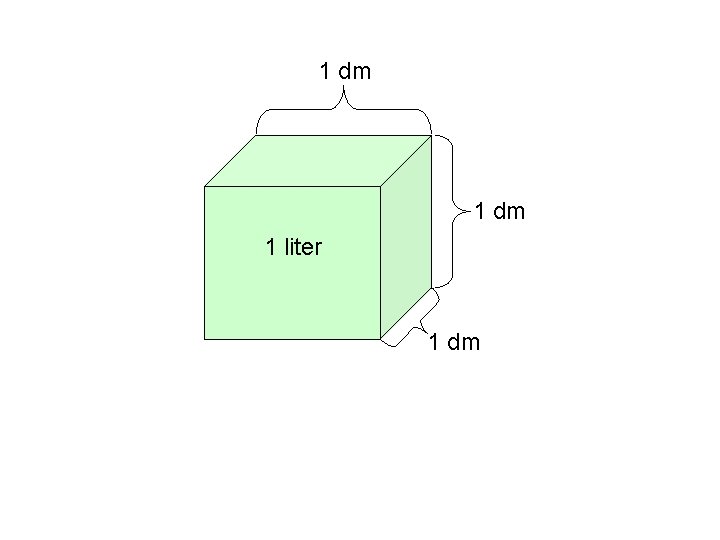
1 dm 1 liter 1 dm

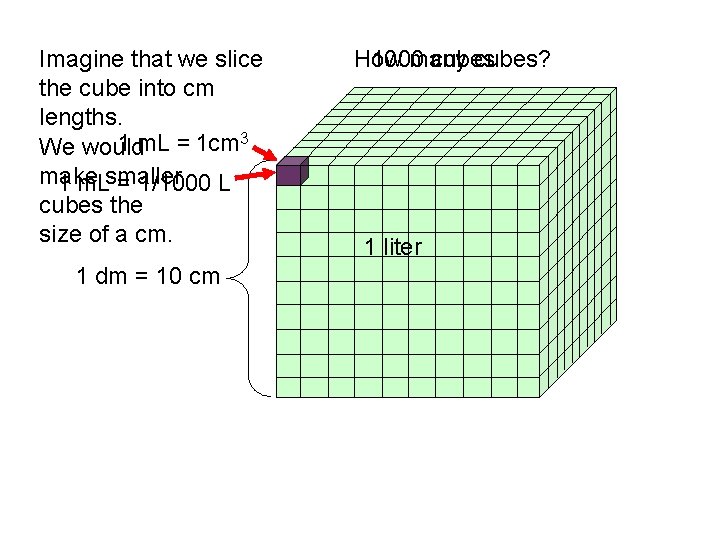
Imagine that we slice the cube into cm lengths. 1 m. L = 1 cm 3 We would make 1 m. Lsmaller = 1/1000 L cubes the size of a cm. 1 dm = 10 cm How 1000 many cubes? 1 liter

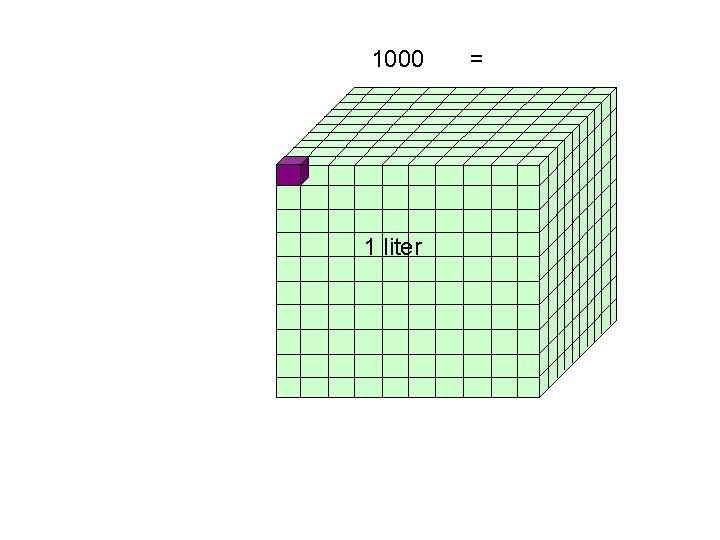
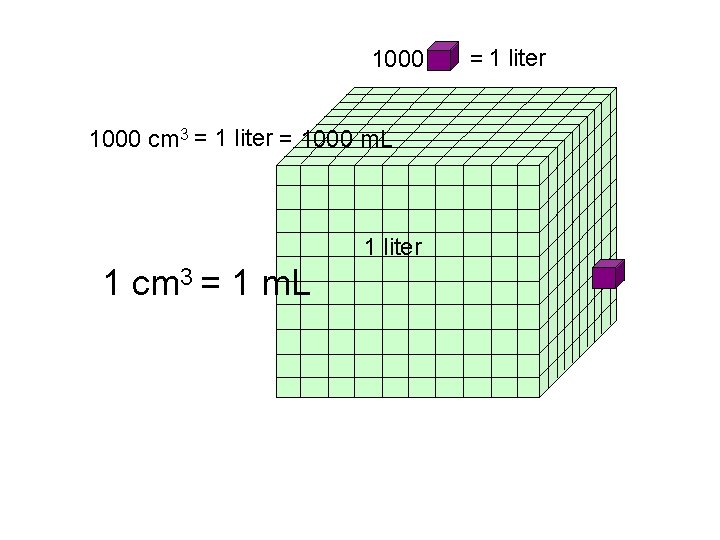
1000 1 liter =

1000 cm 3 = 1 liter = 1000 m. L 1 liter 1 cm 3 = 1 m. L = 1 liter

It’s important that you know what a cm 3 is. And that a cm 3 is also equal to a m. L. Because density is the mass of 1 cm 3 (or 1 m. L) of a substance. 1 cm 3 = 1 m. L = 1/1000 L
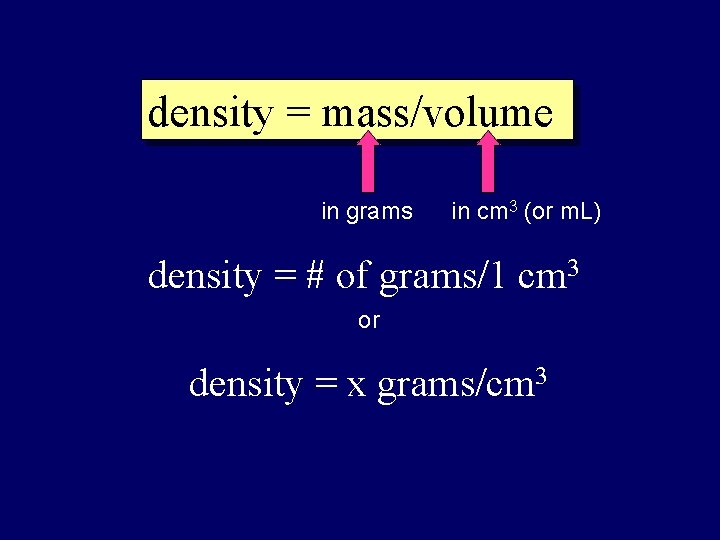
density = mass/volume in grams in cm 3 (or m. L) density = # of grams/1 cm 3 or density = x grams/cm 3

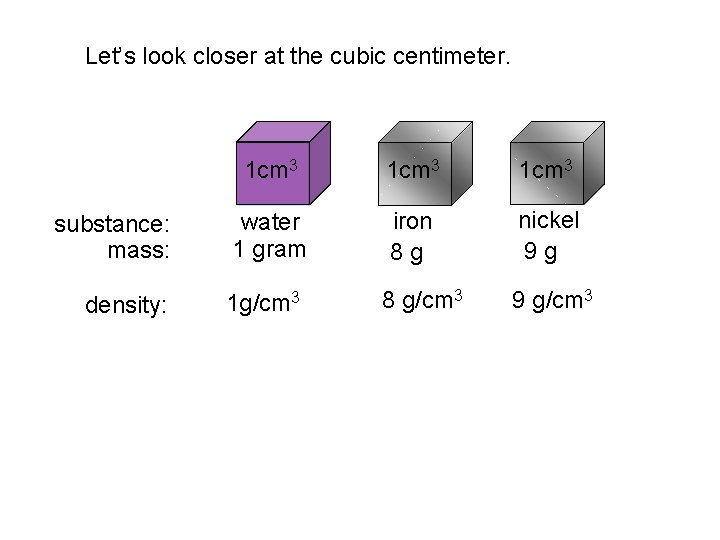
Let’s look closer at the cubic centimeter. 1 cm 3 substance: mass: water 1 gram iron 8 g nickel 9 g density: 1 g/cm 3 8 g/cm 3 9 g/cm 3

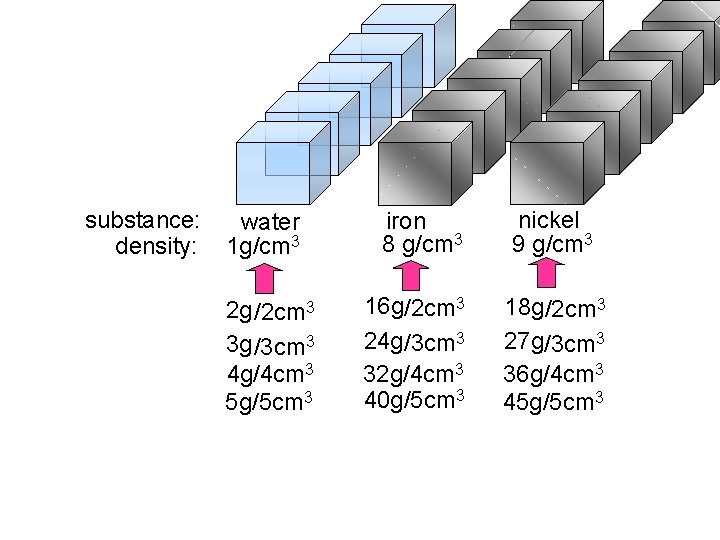
substance: density: water 1 g/cm 3 2 g /2 cm 3 3 g /3 cm 3 4 g/4 cm 3 5 g/5 cm 3 iron 8 g/cm 3 16 g/2 cm 3 24 g/3 cm 3 32 g/4 cm 3 40 g/5 cm 3 nickel 9 g/cm 3 18 g/2 cm 3 27 g/3 cm 3 36 g/4 cm 3 45 g/5 cm 3

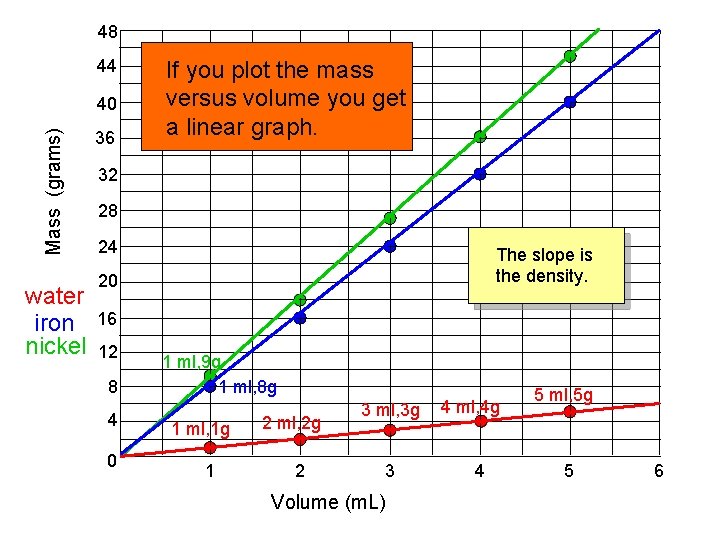
48 44 Mass (grams) 40 water iron nickel 36 If you plot the mass versus volume you get a linear graph. 32 28 24 The slope is the density. 20 16 12 8 4 0 1 ml, 9 g 1 ml, 8 g 1 ml, 1 g 1 2 ml, 2 g 2 3 ml, 3 g 3 Volume (m. L) 4 ml, 4 g 4 5 ml, 5 g 5 6
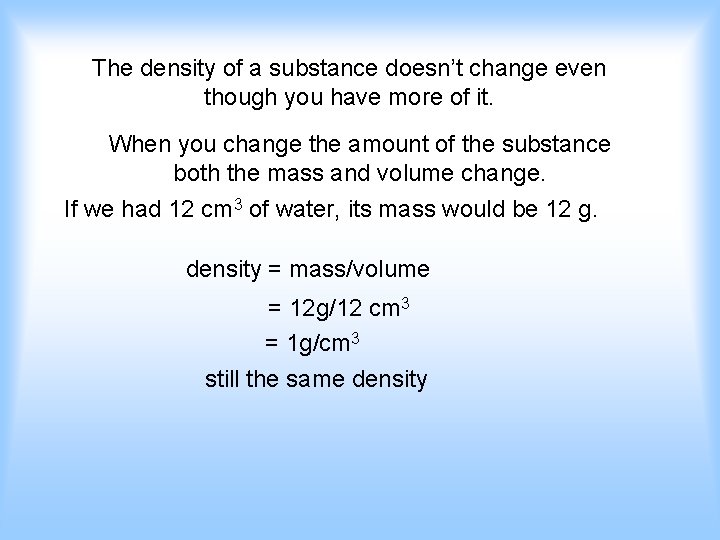
The density of a substance doesn’t change even though you have more of it. When you change the amount of the substance both the mass and volume change. If we had 12 cm 3 of water, its mass would be 12 g. density = mass/volume = 12 g/12 cm 3 = 1 g/cm 3 still the same density

Sometimes you can identify a metal by its density. For example, suppose we had a chunk of metal and we weren’t sure whether it was iron or nickel. They look the same. How can we tell whether the metal is nickel or iron? density of iron = 8 g/m. L density of nickel = 9 g/ml If we can determine the density of the unknown metal, we can tell whether it’s iron or nickel.

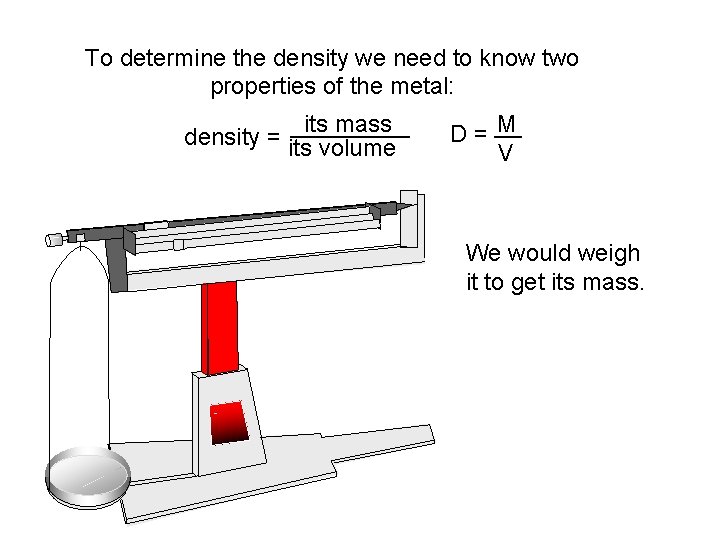
To determine the density we need to know two properties of the metal: its mass _____ density = its volume M D = __ V We would weigh it to get its mass.

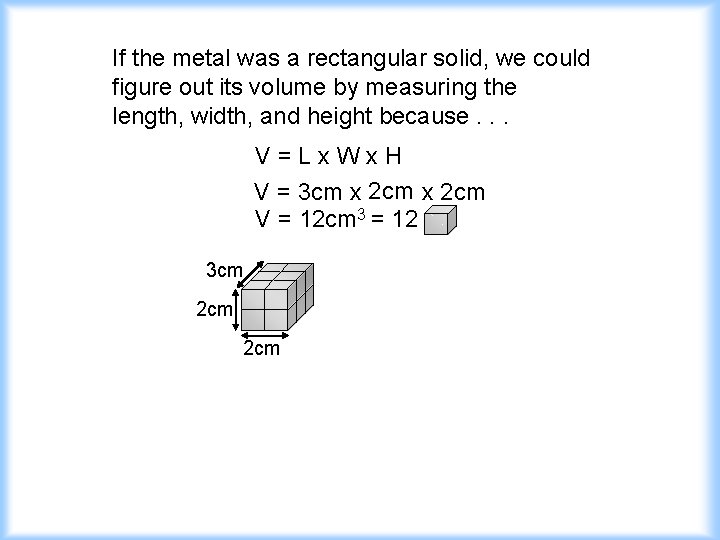
If the metal was a rectangular solid, we could figure out its volume by measuring the length, width, and height because. . . V=Lx. Wx. H V = 3 cm x 2 cm V = 12 cm 3 = 12 3 cm 2 cm

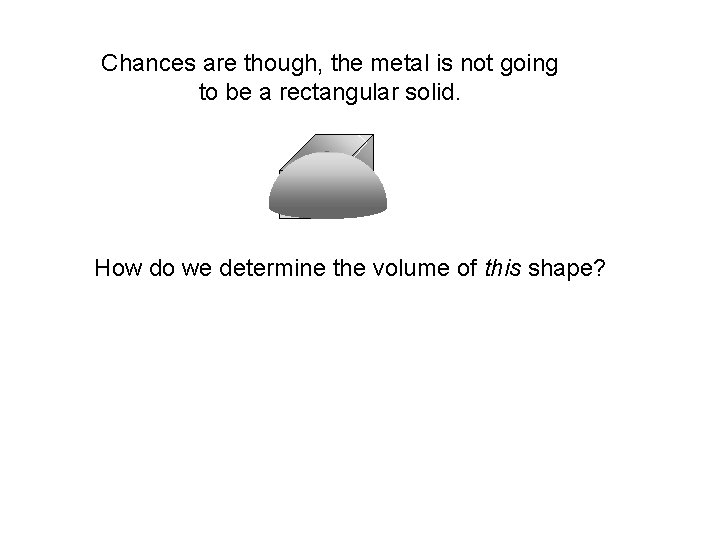
Chances are though, the metal is not going to be a rectangular solid. How do we determine the volume of this shape?

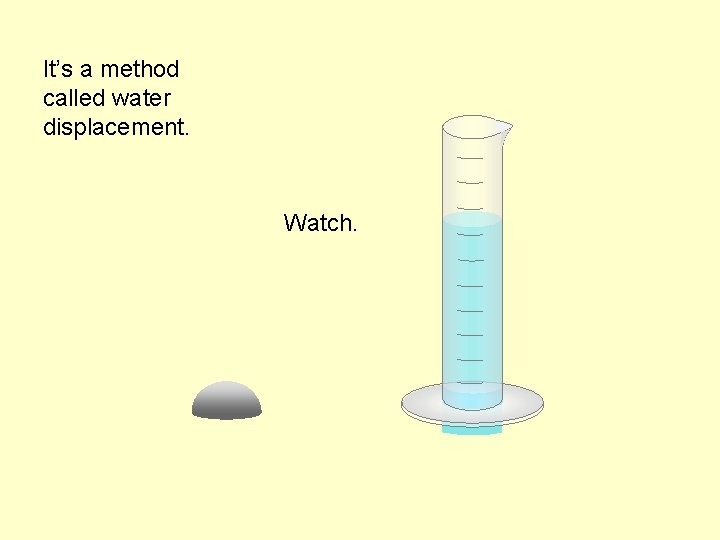
It’s a method called water displacement. Watch.

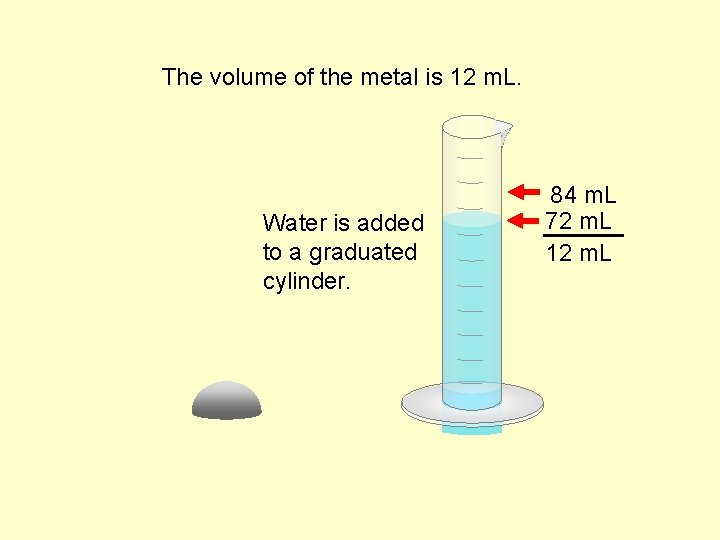
The volume of the metal is 12 m. L. Water is added to a graduated cylinder. 84 m. L 72 m. L 12 m. L

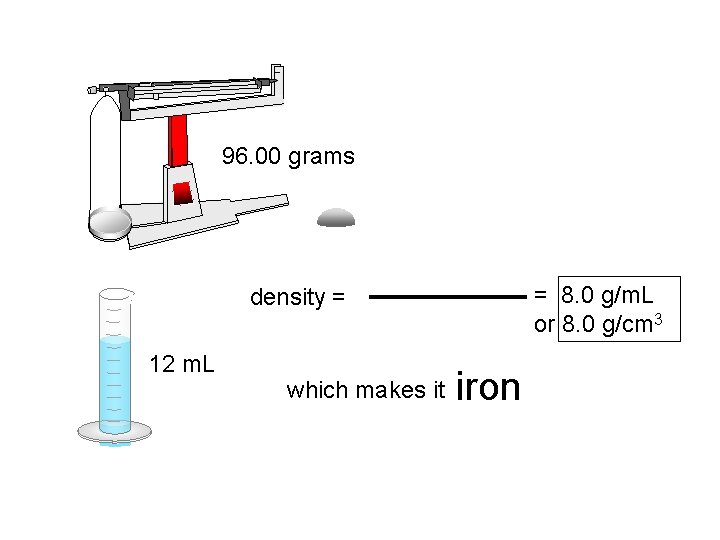
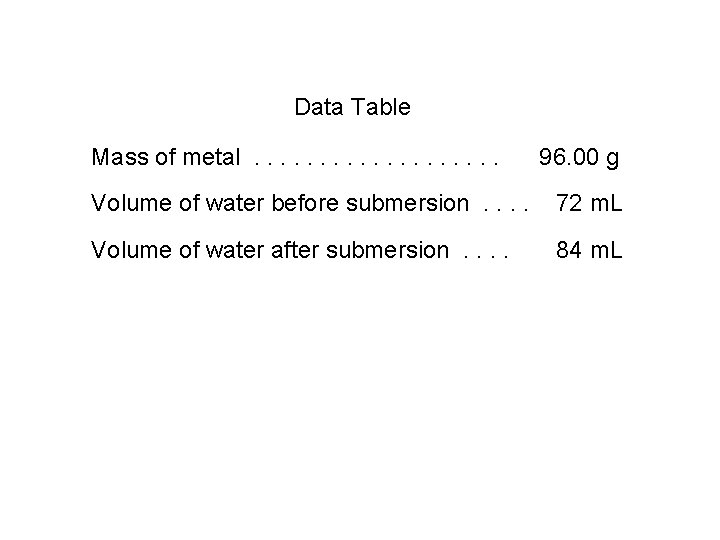
96. 00 grams = 8. 0 g/m. L or 8. 0 g/cm 3 density = 12 m. L which makes it iron

Data Table Mass of metal. . . . . 96. 00 g Volume of water before submersion. . 72 m. L Volume of water after submersion. . 84 m. L