Zeta potential measurement Introduction Zeta potential is the




- Slides: 11

Zeta potential measurement

Introduction • Zeta potential is the charge that is located at the slipping point of a particle in a medium. • Zeta potential is used mostly in Colloidal chemistry. • If the magnitude of zeta potential is > 30 m. V then it is in stable suspension • p. H has a major effect on the system • Zeta potential can not be calculated directly. • However using Electro kinetic effects zeta potential can be measured

Zeta Potential system(general) • He/Ne laser, goes to a beam splitter which then sends the beam to a fixed mirror and a modulator. • Then the beams head into the sample with an electrode on both ends. • Then a photomultiplier picks up the signal and sends it to a computer.

Electro Kinetic Effects • Electro kinetic potential is caused by the interfacial double layer within a solution. • The double layer has a stern layer and a diffuse layer. • Using an electric field either electrophoresis or electro osmosis will cause the particles to move

Electrophoresis • Under an applied electric field the movement of charged particles in relation to the liquid its suspended in is measured • The velocity of the movement depends on the strength of the field, dielectric constant, viscocity, and zeta potential • Using Henry’s law zeta potential can be measured

Electrophoresis (Cont. ) • Measurement of electrophoretic movement • Laser Doppler Velocimetry(LDV)

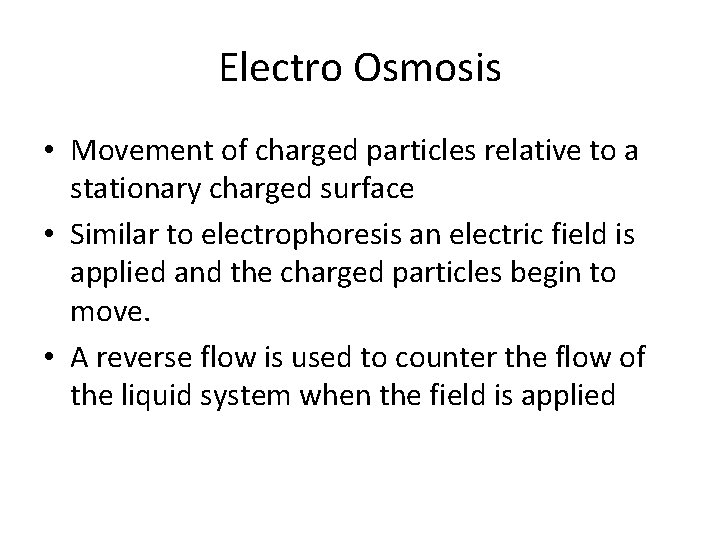
Electro Osmosis • Movement of charged particles relative to a stationary charged surface • Similar to electrophoresis an electric field is applied and the charged particles begin to move. • A reverse flow is used to counter the flow of the liquid system when the field is applied

Electro Osmosis (Cont. ) • One problem is that there is only two places where there is truly the electro osmosis effect • M 3 measurment-Using LDV • Slow Field Reversal (SFR) • Fast Field Reversal (FFR)

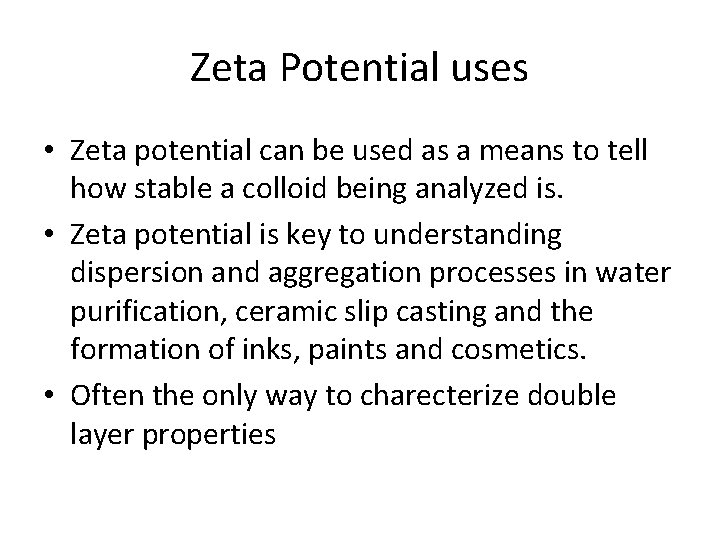
Zeta Potential uses • Zeta potential can be used as a means to tell how stable a colloid being analyzed is. • Zeta potential is key to understanding dispersion and aggregation processes in water purification, ceramic slip casting and the formation of inks, paints and cosmetics. • Often the only way to charecterize double layer properties

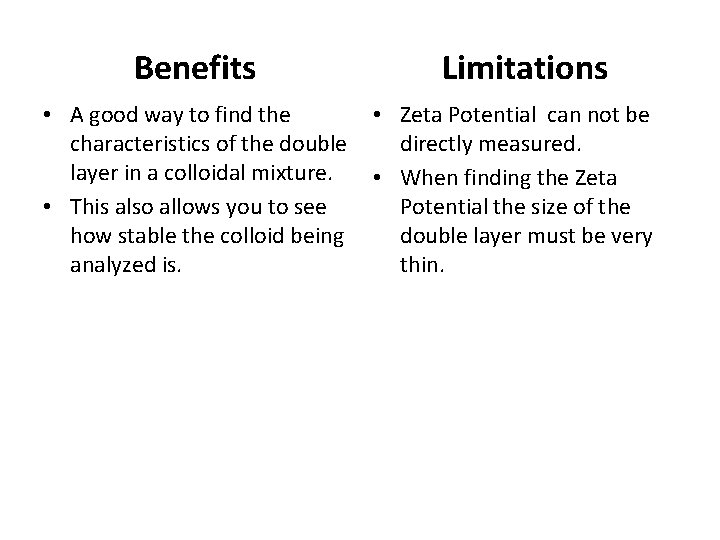
Benefits • A good way to find the characteristics of the double layer in a colloidal mixture. • This also allows you to see how stable the colloid being analyzed is. Limitations • Zeta Potential can not be directly measured. • When finding the Zeta Potential the size of the double layer must be very thin.

Works Cited • Zeta Potential Overview, Brookhaven Instruments, 1, 2009 • Lyklema, J. Fundamentals of Interface and Colloid Science, vol. 2, page. 3. 208, 1995 • The Zeta Potential, Colloidal Dynamics, 4, 1999 • Zeta Potential Introduction, Nicomp 380 ZLS, 101, 2006 • IUPAC Technical Support. Measurement and Interpretation of Electrokinetic Phenomena, vol. 77, pp. 1753 -1805. 2005 • Zeta Potential theory, pp. 16. 1 -16. 12. 2005 • http: //www. silver-colloids. com/Tutorials/Intro/pcs 1. html
 Osmotic potential vs water potential
Osmotic potential vs water potential Flaccid turgid and plasmolysis
Flaccid turgid and plasmolysis ψs
ψs Graded potential vs action potential
Graded potential vs action potential Graded potentials
Graded potentials Graded vs action potential
Graded vs action potential Graded potential vs action potential
Graded potential vs action potential Refractory period neuron
Refractory period neuron Water potential
Water potential Source of bioelectric potential is
Source of bioelectric potential is End-plate potential vs action potential
End-plate potential vs action potential End plate potential
End plate potential





















