Worlds most famous last words Hey watch this







- Slides: 12

World’s most famous last words: “Hey, watch this!”

Rather than atoms losing and gaining electrons to form compounds, compounds can also be formed when atoms share electrons. The resulting bonds are called covalent bonds. When this happens, molecules are formed. “A molecule is an assembly of two or more atoms tightly bound together. ” - Brown and Le. May The molecule behaves as a single, distinct object.

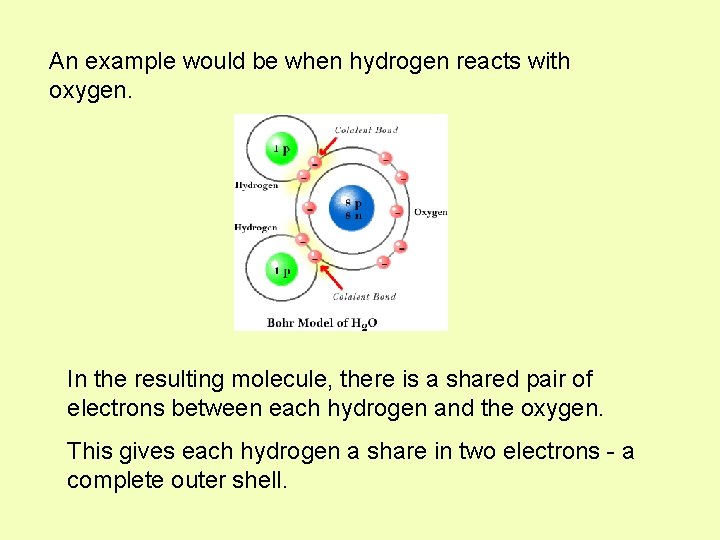
An example would be when hydrogen reacts with oxygen. In the resulting molecule, there is a shared pair of electrons between each hydrogen and the oxygen. This gives each hydrogen a share in two electrons - a complete outer shell.

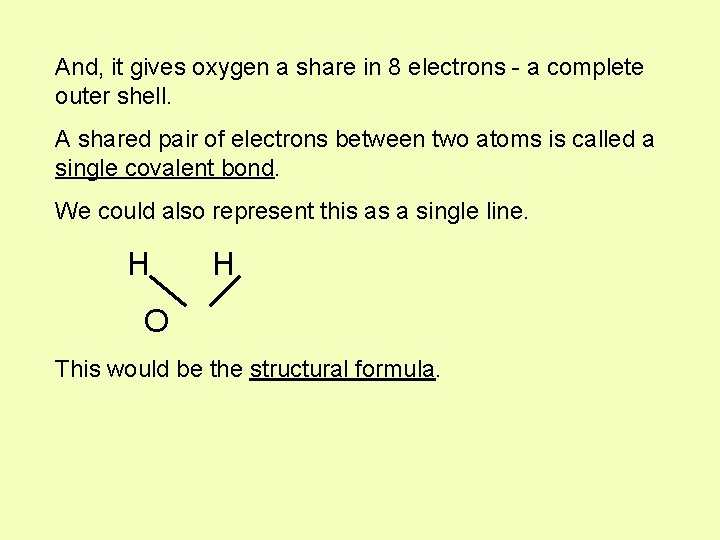
And, it gives oxygen a share in 8 electrons - a complete outer shell. A shared pair of electrons between two atoms is called a single covalent bond. We could also represent this as a single line. H H O This would be the structural formula.

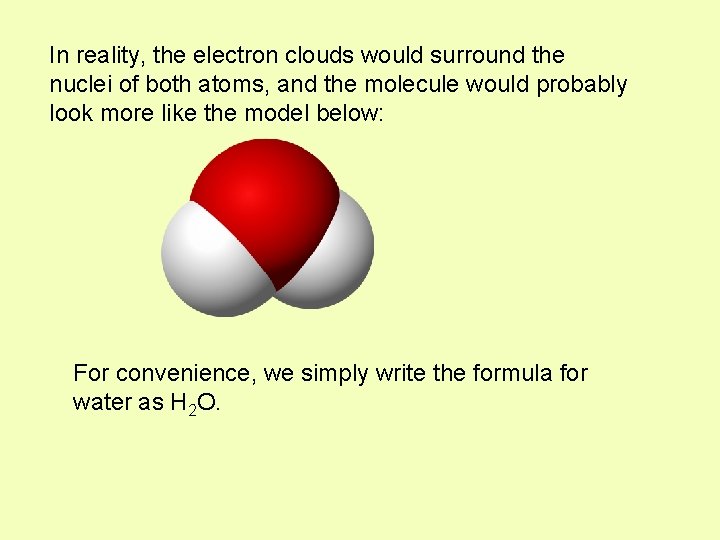
In reality, the electron clouds would surround the nuclei of both atoms, and the molecule would probably look more like the model below: For convenience, we simply write the formula for water as H 2 O.

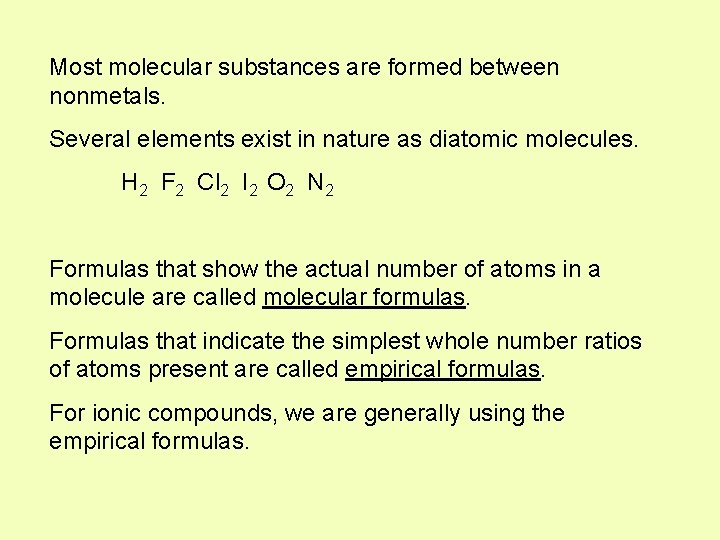
Most molecular substances are formed between nonmetals. Several elements exist in nature as diatomic molecules. H 2 F 2 Cl 2 I 2 O 2 N 2 Formulas that show the actual number of atoms in a molecule are called molecular formulas. Formulas that indicate the simplest whole number ratios of atoms present are called empirical formulas. For ionic compounds, we are generally using the empirical formulas.

CHARACTERISTICS OF COVALENT COMPOUNDS 1. Have low melting points and boiling points - many would be gases or liquids at room temperature. 2. Would not conduct electricity. 3. Many are not soluble in water or other polar solvents.

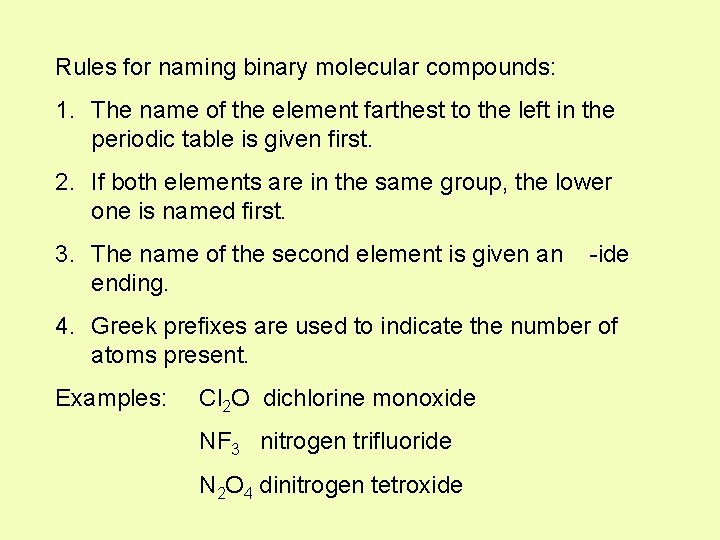
Rules for naming binary molecular compounds: 1. The name of the element farthest to the left in the periodic table is given first. 2. If both elements are in the same group, the lower one is named first. 3. The name of the second element is given an ending. -ide 4. Greek prefixes are used to indicate the number of atoms present. Examples: Cl 2 O dichlorine monoxide NF 3 nitrogen trifluoride N 2 O 4 dinitrogen tetroxide

Name the following compounds: a. SO 2 b. PCl 5 c. N 2 O 3 Give the chemical formula for: a. Silicon tetrabromide b. Disulfur dichloride

ACIDS For the time being, acids are defined as substances that yield hydrogen ions (H+) when dissolved in water. For compounds that end in -ide, the ending will change to -ic. HCl hydrogen chloride (gas) HCl hydrochloric acid as a water solution H 2 S hydrogen sulfide (gas) H 2 S hydrosulfuric acid as a water solution HCN hydrogen cyanide (gas) HCN hydrocyanic acid as a water solution

Other common acids are: H 2 SO 4 sulfuric acid HNO 3 nitric acid H 3 PO 4 phosphoric acid H 2 CO 3 carbonic acid

Bases will be defined, for the time being, as substances that yield hydroxide ion (OH-) when dissolved in water. Some common bases are: Na. OH sodium hydroxide KOH potassium hydroxide Ca(OH)2 calcium hydroxide NH 4 OH ammonium hydroxide Note: when you react an acid with a base, the products will be water (H+ + OH- --> H 2 O) and a salt. HCl + Na. OH --> H 2 O + Na. Cl
 Song with hey in chorus
Song with hey in chorus Worlds together worlds apart 4th edition
Worlds together worlds apart 4th edition Why why why why
Why why why why Hey hey you you get off of my cloud
Hey hey you you get off of my cloud Hey hey you you get off of my cloud
Hey hey you you get off of my cloud Hey hey people
Hey hey people Laughing and a running hey hey
Laughing and a running hey hey Words change worlds
Words change worlds Most famous czech composers
Most famous czech composers What is the most famous sermon ever preached?
What is the most famous sermon ever preached? Most famous epics
Most famous epics What is the most famous paradox?
What is the most famous paradox? William shakespare
William shakespare