Thermochemical Equations Using the grid to solve for


- Slides: 6

Thermochemical Equations Using the grid to solve for desired quanties

Recap • An exothermic change is a reaction that releases energy: energy can be thought of as a product • An endothermic change is one in which the energy must be added for the reaction to occur: energy can be thought of as a reactant • Enthalpy (H) is the energy (heat) content of a system at constant pressure. You cannot measure the actual energy or enthalpy of a substance, but you can measure the change in enthalpy ∆Hrxn.

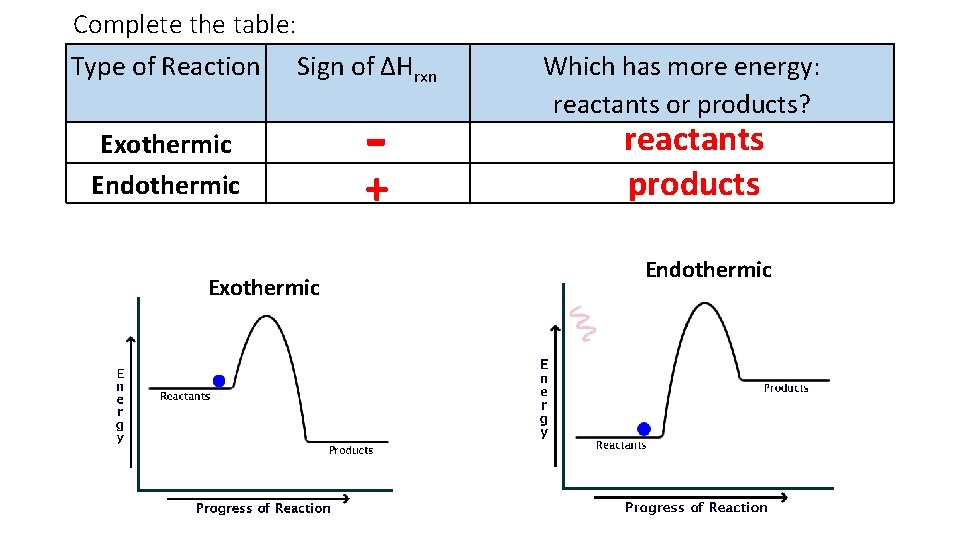
Complete the table: Type of Reaction Sign of ∆Hrxn Exothermic Endothermic Exothermic - + Which has more energy: reactants or products? reactants products Endothermic

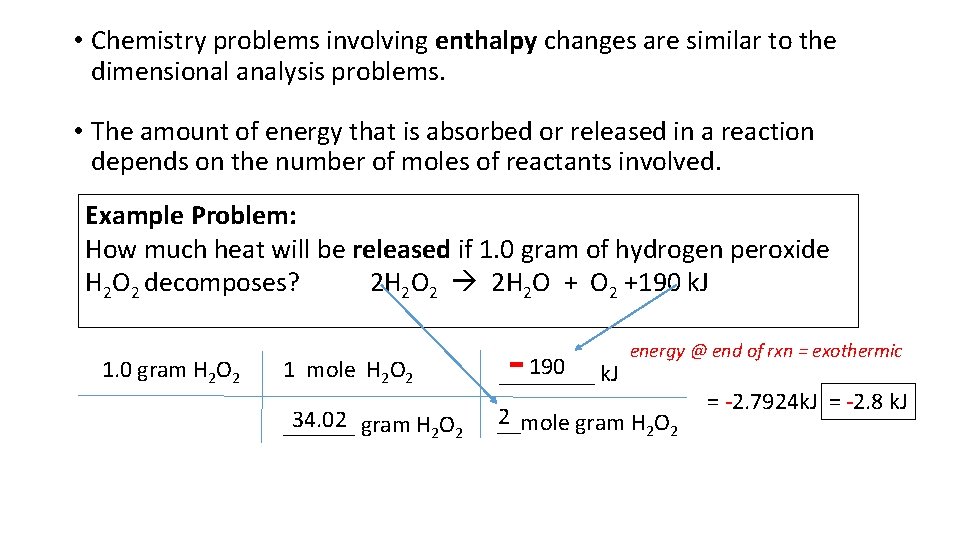
• Chemistry problems involving enthalpy changes are similar to the dimensional analysis problems. • The amount of energy that is absorbed or released in a reaction depends on the number of moles of reactants involved. Example Problem: How much heat will be released if 1. 0 gram of hydrogen peroxide H 2 O 2 decomposes? 2 H 2 O 2 2 H 2 O + O 2 +190 k. J 1. 0 gram H 2 O 2 - energy @ end of rxn = exothermic 1 mole H 2 O 2 190 ____ k. J 34. 02 ______ gram H 2 O 2 2 __mole gram H 2 O 2 = -2. 7924 k. J = -2. 8 k. J

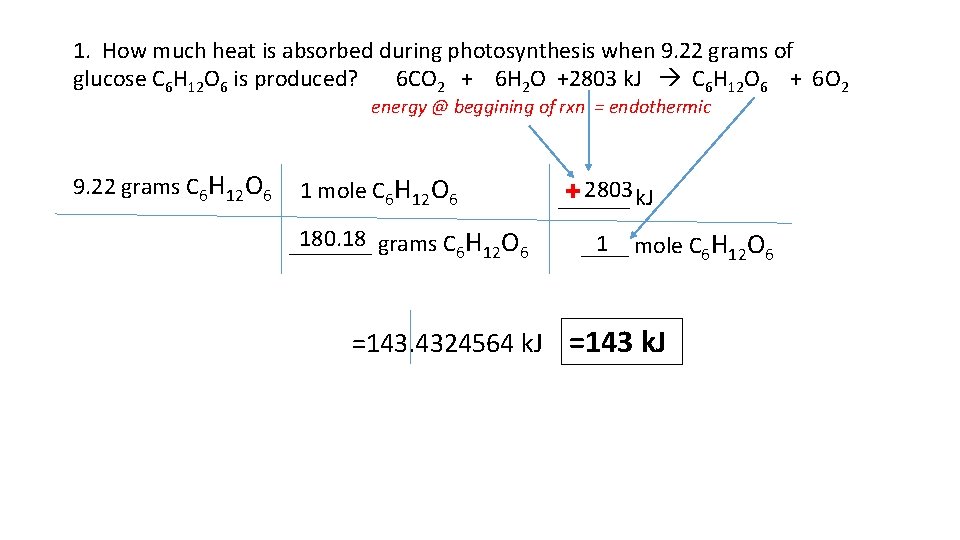
1. How much heat is absorbed during photosynthesis when 9. 22 grams of glucose C 6 H 12 O 6 is produced? 6 CO 2 + 6 H 2 O +2803 k. J C 6 H 12 O 6 + 6 O 2 energy @ beggining of rxn = endothermic 9. 22 grams C 6 H 12 O 6 1 mole C 6 H 12 O 6 180. 18 _______ grams C 6 H 12 O 6 + 2803 ______ k. J 1 ____ mole C 6 H 12 O 6 =143. 4324564 k. J =143 k. J

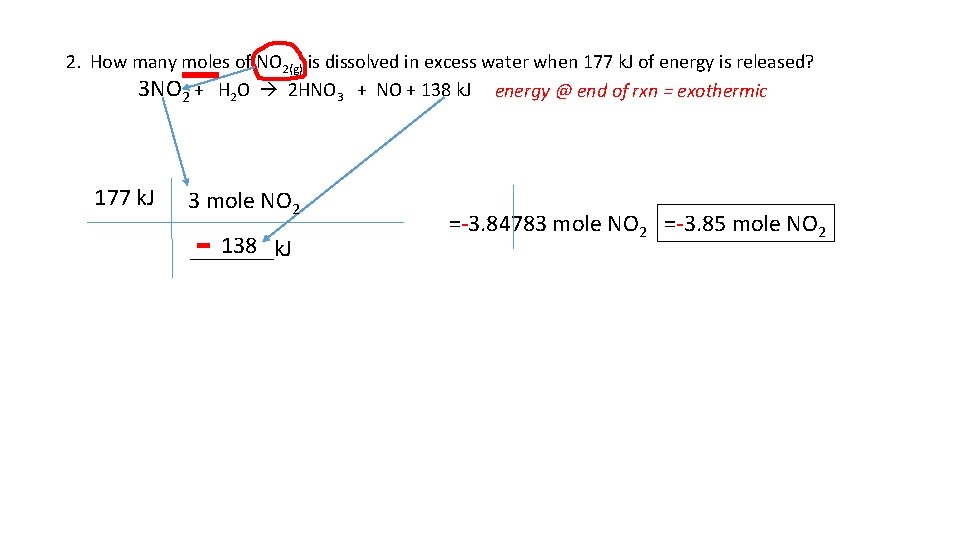
2. How many moles of NO 2(g) is dissolved in excess water when 177 k. J of energy is released? 3 NO 2 + H 2 O 2 HNO 3 + NO + 138 k. J energy @ end of rxn = exothermic 177 k. J 3 mole NO 2 - 138 _______k. J =-3. 84783 mole NO 2 =-3. 85 mole NO 2